Physical properties of halogens
advertisement

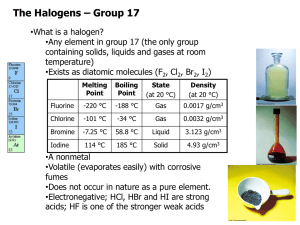
Physical properties of halogens Electronic configurations • Halogens are in group VII of the periodic table and so have seven electrons in their outer shell. • F 1s2 2s2 2p5 • Cl 1s2 2s2 2p5 3s2 3p5 • Br 1s2 2s2 2p5 3s2 3p5 4s2 (3d10) 4p5 • I 1s2 2s2 2p5 3s2 3p5 4s2 (3d10) 4p5 5s2 (4d10) 5p5 Bonding • Halogens require one electron to complete their outer shell. • This can be obtained by gaining an electron to form a halide anion. • Eg: Cl + e → Cl • Alternatively they can share electrons in a single covalent bond; • Clx + Hx → ClxxH Diatomic molecules • The halogens are found in their native states as diatomic molecules. • F:F • Cl:Cl • Br:Br • I:I • Halogens form simple molecular lattices held together by weak van der Waals forces. Chlorine is a pale green gas at room temp and pressure. Bromine is an orange brown liquid at room temp and pressure. It has a low BP and is highly volatile. Iodine is a grey, silvery solid at room temperature and pressure. Iodine will not melt, on heating it sublimes, forming a purple gas. MPs and BPs F Cl Br I MP (oC) -220 -101 -7 113 BP (oC) -188 -35 59 Plot the MPs and BPs and explain the trend in terms of structure/bonding. MPs and BPs of halogens • The MPs and BPs increase down the group. • This is because there are more electrons in the atoms. • Which means that the van der Waals forces between molecules become stronger. • So more energy is required to break them and melt or boil the sample. Solubility of halogens Organic layer non-polar solvent. Halogens are more soluble in non-polar than in polar solvents. Aqueous layer polar solvent The bond between atoms is not polarised. Bromine water shaken with hexane. Solubility of chlorine Chlorine is not very soluble in water, but reacts to form pale green chlorine water. Chlorine is more soluble in organic solvents. Aqueous layer Solubility of bromine Bromine readily dissolves in water to give an orange brown solution. Bromine is more soluble in organic solvents. Solubility of iodine Iodine is not very soluble in water. But it is much more soluble in KI, giving a yellow brown solution. Iodine is much more soluble in organic solvents. Organic layer Aqueous layer