Term Test 3
advertisement

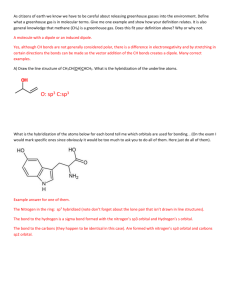
Term Test 3B Instructor: Dr. Robert Davis Chemistry 1050 Name: _____________________ Wednesday, November 17, 2011 ANSWER THE TEST QUESTIONS IN THE SPACE PROVIDED. MARKS WILL BE DEDUCTED FOR INCORRECT USE OF SIGNIFICANT FIGURES AND FOR LACK OF UNITS IN CALCULATIONS. Marking Scheme Question Value Part A: Short Answer Questions A1 – A4 12 Part B: Longer Answer Questions B1 12 B2 7 B3 4 B4 4 B5 3 B6 1 B7 2 Total 45 % Grade 1 Mark Part A: Short Answer Questions [Value] [3] [3] [2] [4] A1. A2. A3. A4. Define or explain (a) anti-bonding molecular orbital (b) sigma bond (c) formal charge Give an example of (a) a tetrahedrally shaped molecule _________________________ (b) a trigonal pyramidal molecule _______________________ (c) a see saw shaped molecule __________________________ List the following molecules in order of decreasing bond polarity (a) PCl3, PBr3, PI3 most polar ____ ____ ____ least polar (b) Cl2, HCl, NaCl, ClBr most polar ____ ____ ____ ____ least polar Calculate a ΔHo value for the reaction H2C=CH2(g) + Cl2(g) → H2ClC–CH2Cl(g) using the bond energies: C–H ( 414 kJ mol–1), C=C ( 611 kJ mol–1), Cl–Cl ( 243 kJ mol–1), C–C (347 kJ mol–1), and C–Cl ( 339 kJ mol–1). 2 Part B: Longer Answer Questions [Value] [12] B1. Complete the following table. Species BeF2 SeCl4 Lewis structure Sketch of shape Name of shape Approximate bond angles Indicate if the species is polar or non–polar 3 XeF4 [7] B2. Construct a molecular orbital energy level diagram for the O2+ homonuclear diatomic species. Consider only the valence shell electrons. Determine whether the species is diamagnetic or paramagnetic. Calculate the bond order. B3. (a) Draw two “equivalent” resonance structures for the NO2Cl molecule. (b) What is the bond order in this molecule? (c) What is the formal charge on N in this molecule? . [4] 4 [4] B4. Balance the following oxidation-reduction reaction taking place in acidic solution. Cr2O72–(aq) + Fe2+(aq) [3] [1] B5. B6. → Cr3+(aq) + Fe3+(aq) Give chemical formulas for molecules not containing carbon where the central atom exhibits (a) sp hybridization (b) sp2 hybridization (c) sp3 hybridization Draw a picture representing the shape of one bond in H–C≡C–H (acetylene). 5 [2] B7. Indicate whether a sp, sp2 or sp3 hybridization scheme should be used to describe the bonding at each C, O and N atom in the molecule represented below. O H C C C NH2 6