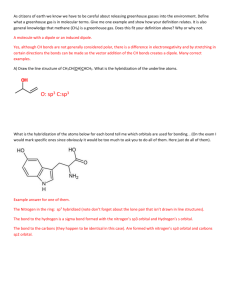
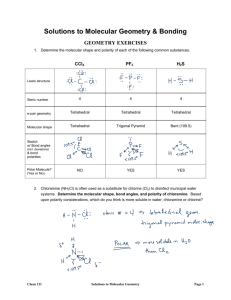
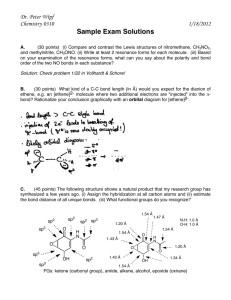
Lewis Structures, VSEPR, Hybridization Worksheet
advertisement
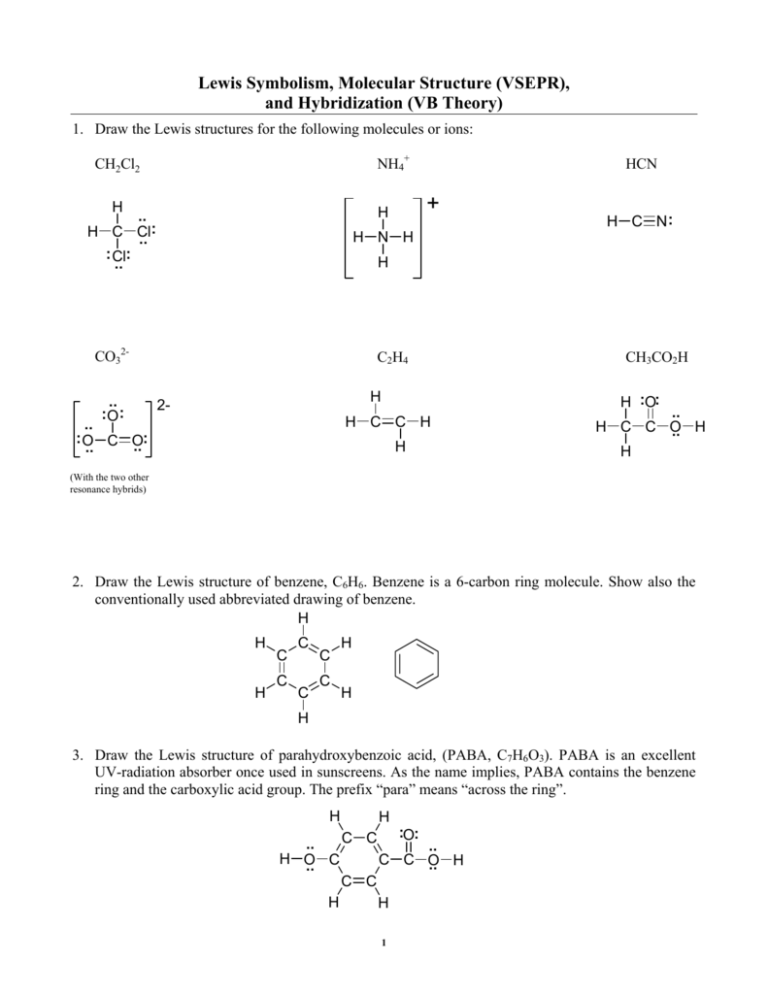
Lewis Symbolism, Molecular Structure (VSEPR), and Hybridization (VB Theory) 1. Draw the Lewis structures for the following molecules or ions: NH4+ CH2Cl2 H H N H H C N: H CO32.. :O : + H .. : H C Cl .. : Cl: .. HCN C2H4 H 2- H :O: H C C H .. : O C O: .. .. CH3CO2H H .. H C C O .. H H (With the two other resonance hybrids) 2. Draw the Lewis structure of benzene, C6H6. Benzene is a 6-carbon ring molecule. Show also the conventionally used abbreviated drawing of benzene. H H H C C C C C C H H H 3. Draw the Lewis structure of parahydroxybenzoic acid, (PABA, C7H6O3). PABA is an excellent UV-radiation absorber once used in sunscreens. As the name implies, PABA contains the benzene ring and the carboxylic acid group. The prefix “para” means “across the ring”. H H C C :O: .. .. H O C C C O .. H .. C C H H 1 5. Two kinds of covalent bonds exist; nonpolar covalent and polar covalent. Describe the carbonhydrogen bond in CH4 as either polar or nonpolar. 6. Draw the Lewis structures of the following molecules or ions. For each, indicate the electronic geometry, molecular geometry, hybridization (if any) of central atoms, and whether the molecule or ion possesses a permanent dipole moment. PO43.. :O: SeO2 3- .. .. :O Se :O .. : .. P .. : O :O .. .. :O .. : This ion may have 1 double bond. electronic geometry N2H4 .. .. H N N H H H This ion shows resonance. __tetrahedral______ ___trigonal planar__ _tetrahedral both N molecular geometry __tetrahedral______ ___bent___________ _trigonal pyramidal hybridization ___sp3 (on P)______ ___sp2 (on Se)_____ _sp3 (both N)_____ Dipole Moment ___no____________ ___yes____________ _yes____________ XeF4 CBr4 .. .. .. : .. F: F .. Xe .. .. : .. : F F .. .. C2H2 Br Br C Br Br H C C H Lone-pairs omitted because I was tired. electronic geometry __octahedral_______ __tetrahedral______ __linear (both C)__ molecular geometry __square planar____ __tetrahedral______ __linear (both C)__ hybridization __sp3d2 (on Xe)____ __sp3 (on C)_______ __sp (on C)_______ Dipole Moment __no_____________ __no_____________ __no____________ 2 NO2.. :O .. N SeO42- O 2- Se O O O O .. : This ion shows resonance. electronic geometry N2F2 .. .. .. .. :.. F N N F ..: :.. :.. F: F: This ion may have 1 or 2 double bonds. ___trigonal planar__ __tetrahedral______ _tetrahedral both N molecular geometry ___bent___________ __tetrahedral______ _trigonal pyramidal hybridization ___sp2 (on N)______ __sp3 (on Se)______ _sp3 (both N)_____ Dipole Moment ___yes____________ __no_____________ _yes____________ CF2Cl2 C2H4 _________________ _________________ ________________ molecular geometry _________________ _________________ ________________ hybridization _________________ _________________ ________________ Dipole Moment _________________ _________________ ________________ SO3 electronic geometry 3 NO electronic geometry PBr5 SF6 _________________ _________________ ________________ molecular geometry _________________ _________________ ________________ hybridization _________________ _________________ ________________ Dipole Moment _________________ _________________ ________________ 7. Shown below is the molecule caffeine. Describe the hybridization of every atom that uses hybridized atomic orbitals for bonding. Determine the approximate bond angles. Also indicate the type of bonds formed between atoms (i.e., σ(sp2-p), π(p-p), etc.) Notice that lone-pairs are omitted but assumed present; this convention is typical of many drawings of organic molecules. O CH3 H3 C N N O N N CH3 Atom 1 2 3 4 5 6 7 8 9 10 11 12 Hybridization sp3 sp3 sp2 sp2 sp2 sp3 sp2 sp3 sp2 sp2 sp3 sp3 Bond Angle on Atom 109.5° ~107° ~120° ~120° ~120° ~107° ~120° 109.5° ~118° ~120° 107° 109.5° H3C O 12 O 1 2 3 N 7 CH3 4 10 6 N 5 CH3 8 4 N 11 N 9