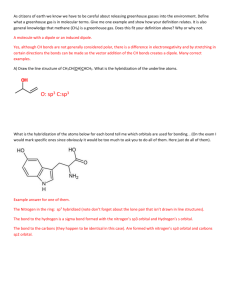
Chapter 11-12 Problems Solutions 1. cis and trans C2H2Cl2 has the
advertisement
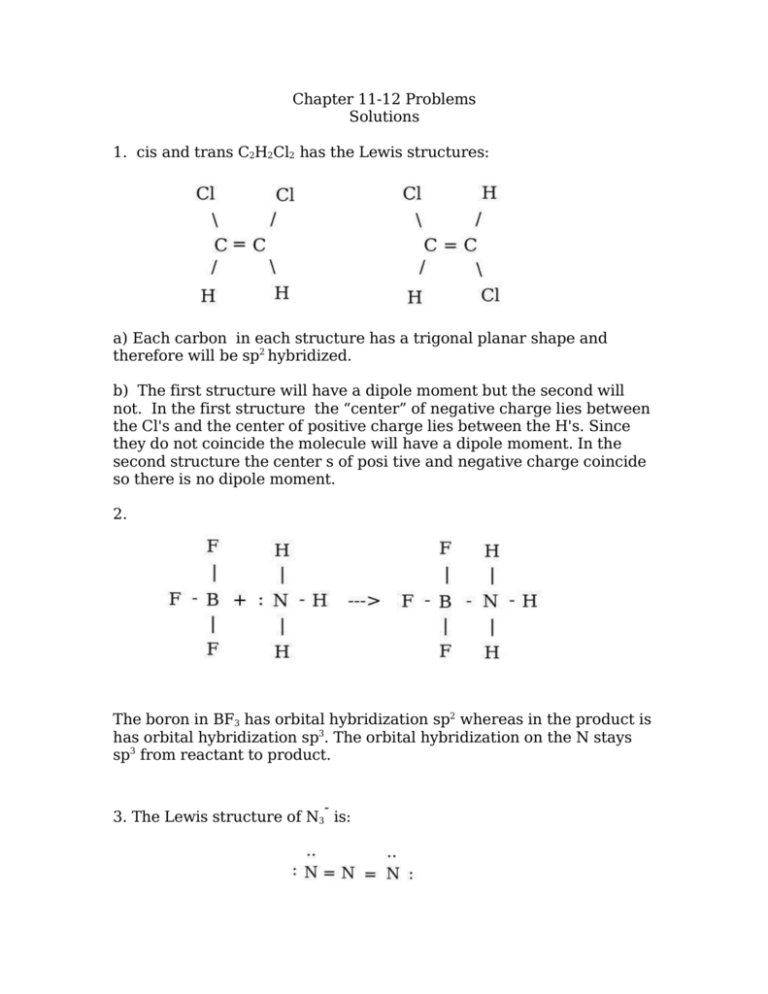
Chapter 11-12 Problems Solutions 1. cis and trans C2H2Cl2 has the Lewis structures: a) Each carbon in each structure has a trigonal planar shape and therefore will be sp2 hybridized. b) The first structure will have a dipole moment but the second will not. In the first structure the “center” of negative charge lies between the Cl's and the center of positive charge lies between the H's. Since they do not coincide the molecule will have a dipole moment. In the second structure the center s of posi tive and negative charge coincide so there is no dipole moment. 2. The boron in BF3 has orbital hybridization sp2 whereas in the product is has orbital hybridization sp3. The orbital hybridization on the N stays sp3 from reactant to product. 3. The Lewis structure of N3 is: For shape purposes, remember that a double or triple bond counts as a single pair of electrons. Therefore, around the central nitrogen atom there are two pairs of electrons, both bonding pairs, which leads to the conclusion that the electron pair geometry is linear. The linear geometry is associated with sp orbital hybridization. 4. Molecule Lewis Structure HSO4- Shape Class Molecular Shape AX4 tetrahedral Orbital Hybridization sp3 BeH2 AX2 linear sp BF4- AX4 tetrahedral sp3 XeOF4 AX5E square planar sp3d2 SbCl5 5. diazomethane: formate anion: sulfur dioxide: sulfuric acid: AX5 trigonal bipyramidal sp3d