Density Calculation Worksheet: Identify Unknown Substances
advertisement
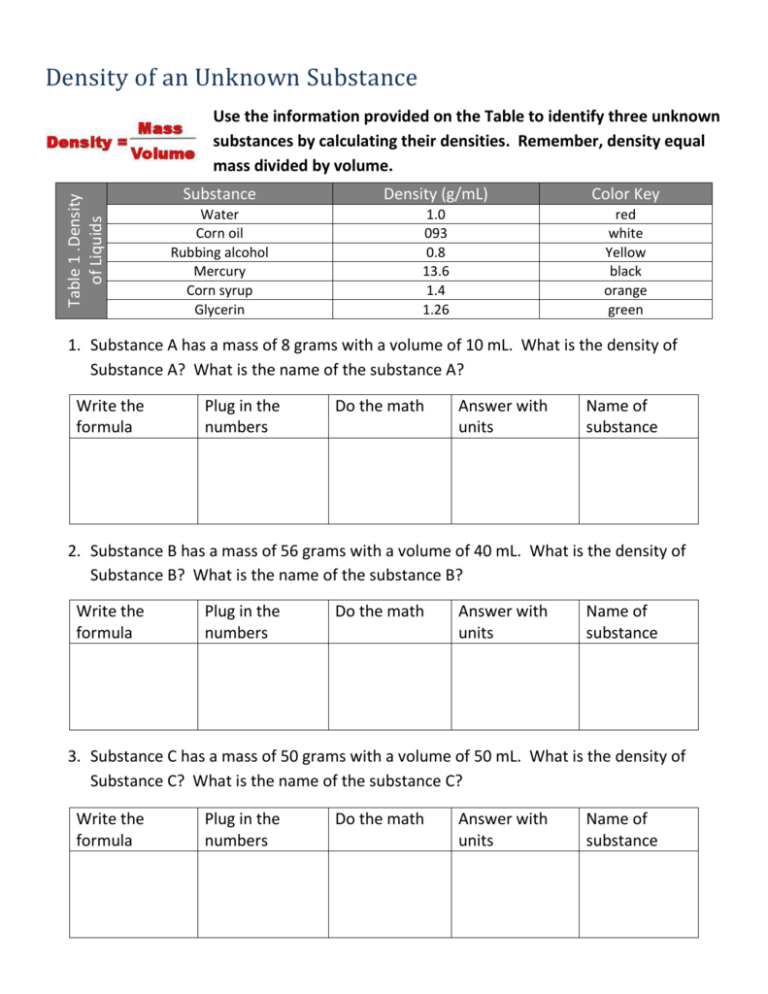
Density of an Unknown Substance Table 1 .Density of Liquids Use the information provided on the Table to identify three unknown substances by calculating their densities. Remember, density equal mass divided by volume. Substance Density (g/mL) Color Key Water Corn oil Rubbing alcohol Mercury Corn syrup Glycerin 1.0 093 0.8 13.6 1.4 1.26 red white Yellow black orange green 1. Substance A has a mass of 8 grams with a volume of 10 mL. What is the density of Substance A? What is the name of the substance A? Write the formula Plug in the numbers Do the math Answer with units Name of substance 2. Substance B has a mass of 56 grams with a volume of 40 mL. What is the density of Substance B? What is the name of the substance B? Write the formula Plug in the numbers Do the math Answer with units Name of substance 3. Substance C has a mass of 50 grams with a volume of 50 mL. What is the density of Substance C? What is the name of the substance C? Write the formula Plug in the numbers Do the math Answer with units Name of substance 4. Create and complete the table below by sequencing the substances from least dense to most dense. (substances A, B, and C) Density of substance (g/mL) Volume of Substance (mL) Name of substance 5. If you were to pour these substances into one graduated cylinder, describe how they would be arranged in the graduated cylinder. 6. Utilize the information in Table 1 to color the graduated cylinder diagram with the appropriate volume of each liquid. Label the name of each Liquid. 7. What two physical properties must you know before you can calculate the density of a substance? 8. What instruments could you use in lab to measure those properties of a liquid?