Name:
advertisement

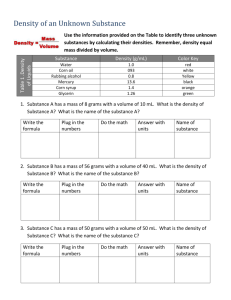
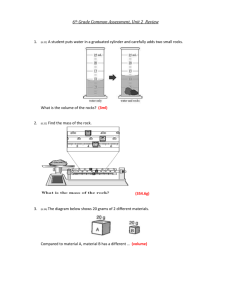
Name: Liquid Density Lab Date: Period: (4 points) ___________________________ Title (4 points) Problem: Which is liquid is more dense: water or the liquid sample brought in _______________? (Fill in the blank for your liquid. 4 points. If Mrs. McNees provides your liquid subtract 4 points) Research: (12 points) Define: liquid: density of water: Citation: (Author, Title, Year published, page) Hypothesis: If ___________________________________________, then ___________________ is more dense because_________________________________________ (Fill in the blanks. 8 points) Procedure: Gather materials: [list materials] (8 points) A. Measure mass and volume of water: [write in numbers and units] (12 points) ______2. mass of water + graduated cylinder 1. Measure mass of graduated cylinder first. 2. Add ten milliliters of the liquid. Record the new mass. -_____1. mass of graduated cylinder only 3. Subtract to find mass of object, Label units ______3. mass of water _____________ B. Measure the mass and volume of the liquid brought in. ______2. mass of liquid + graduated cylinder -_____1. mass of graduated cylinder only ______3. mass of liquid _____________ Observations: Label all units correctly (16 points) Identify Item 1. Mass (g) A. Water B. Conclusions: (12 points) P. H. 2. Volume (mL) 10 mL 10 mL 3. Density (g/mL) Density Drill Some objects tend to be “heavy,” while other objects seem “light.” But unless you are comparing the same volume of each object, these descriptions have little value. And that is where the concept of density comes in. For density refers to how much mass an object has in a particular volume. Scientifically, density is described as mass per unit volume, or Density = mass/volume. Because mass is measured in grams and volume is measured in cubic centimeters, the unit for density is grams per cubic centimeter. If the mass and volume of an object are known, its density can be determined by dividing the volume value into mass value. Similarly, if the density and mass are known, the object’s volume can be determined by dividing the density value into the mass value. Finally, if an object’s density and volume are known, its mass can be found by multiplying these two values. You can see how density, mass and volume are related by doing this activity. In each situation, you are given enough information to determine the unknown value in the formula D = M/V