LIQUID DENSITY LAB
advertisement

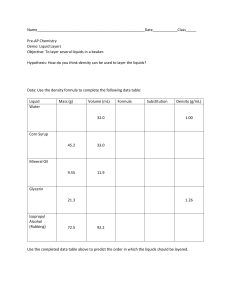
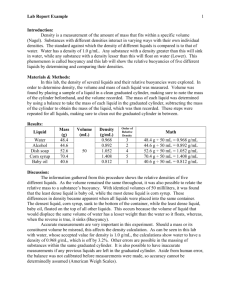
LIQUID DENSITY LAB (Page 107) Step 1: Take the mass of the first graduated cylinder so you can minus it out. Step 2: Measure out 20 ml of water. Remember that 20 ml = 20 cm3 Step 3: Take the mass of the water. ________ g Step 4: Using a clean graduated cylinder, zero out the balance. Step 5: Measure out the mass of 20 ml of corn oil. ________ g Step 6: Measure out the mass of 20 ml of maple syrup. _________ g. Step 7: Pour the contents of the three liquids into the plastic cup. Draw what you see Step 8: Put the foam plate on top of the cup. Step 9: Flip the cup while holding the plate firmly on top to avoid making a mess. Record what you see. Step 10: Calculate the Density of the Four Liquids. Density = Mass/Volume. Quick reminder - Volume is how much Water: Density = Mass (_________ g) / Volume (_________ml) = __________ g/ml Corn Oil: Density = Mass (_________ g) / Volume (_________ml) = __________ g/ml Maple Syrup: Density = Mass (_________ g) / Volume (_________ml) = __________ g/ml Reflection: Why do you think the liquids behaved the way they did? Is there a link between the densities and the order of the levels?