Calculating Atomic Masses
advertisement

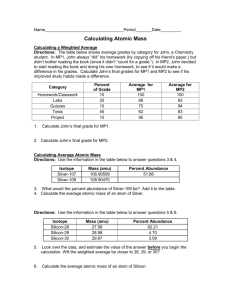
Calculating Atomic Masses Isotopes are atoms of the same atomic number having different masses due to a different number of neutrons. The atomic mass of an element is the weighted average of the masses of the isotopes of that element. The weighted average takes into account both the mass and relative abundance of each isotope as it occurs in nature. To determine Sig Digs, look at % abundances, which are measured values o Significant Digits are associated with measurements or the result of a calculation using measurements. All measurements have some degree of uncertainty, so when we record a measurement or calculation result using measurements we include all digits known with certainty plus the rightmost digit which has some uncertainty. (estimated) FORMULA: The calculation of the average atomic mass of an atom is performed using the relative abundance data from the isotope of each atom. Average atomic mass = [(% abundance of isotope)(mass of isotope)] + [(% abundance of isotope)(mass of isotope)] + [….] 100 EXAMPLE #1 Boron has two common isotopes, Boron-10 which has a mass of 10.013 amu and a relative abundance of 19.9%, and Boron-11 which has an abundance of 80.1% and a mass of 11.009 amu. Calculate the atomic mass of boron. Average atomic mass = [(19.90%)(10.013)] + [(80.10%)(11.009)] 100 = 10.81 amu EXAMPLE #2 Oxygen has three common isotopes, Oxygen-16 which has a mass of 15.995 amu and a relative abundance of 99.763%, Oxygen-17 which has a mass of 16.999 amu and a relative abundance of 0.03750%, and Oxygen-18 which has a mass of 17.999 amu and a relative abundance of 0.1995%. Calculate the atomic mass of oxygen. Average atomic mass = [(99.763%)(15.995)] + [(0.03750%)(16.999)] + [(0.1995%)(17.999)] = 16.00 amu 100