Calculating Average Atomic Mass
advertisement
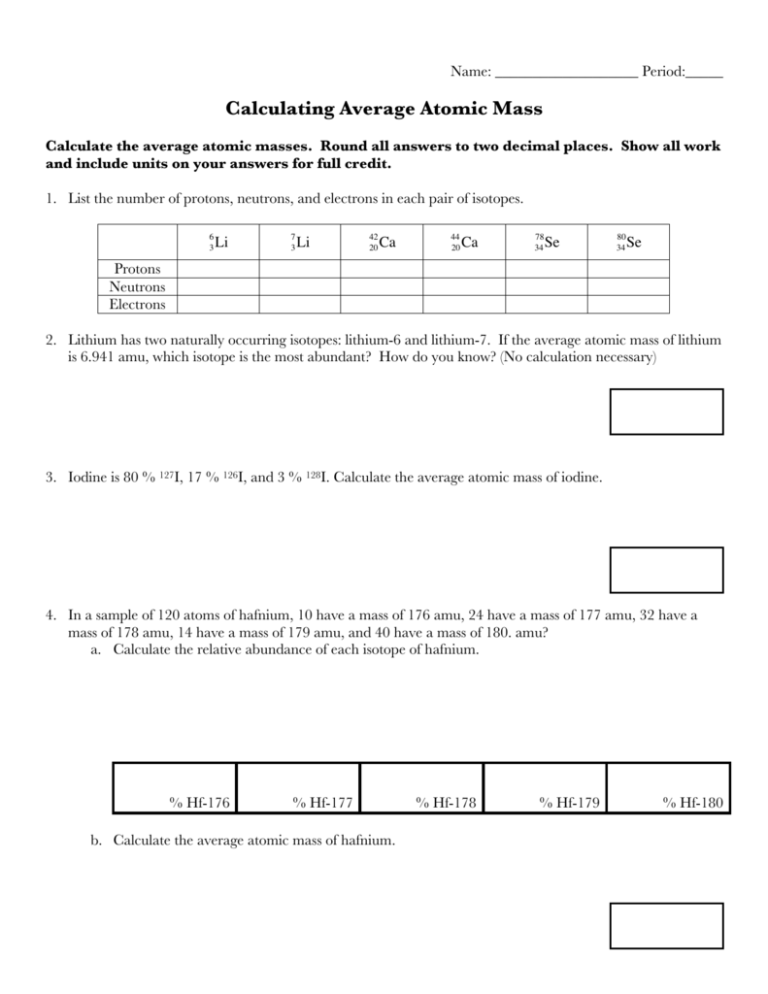
Name: ___________________ Period:_____ Calculating Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. Show all work and include units on your answers for full credit. 1. List the number of protons, neutrons, and electrons in each pair of isotopes. 6 3 Protons Neutrons Electrons € Li € 7 3 Li € 42 20 Ca € 44 20 Ca € 78 34 Se 80 34 Se € 2. Lithium has two naturally occurring isotopes: lithium-6 and lithium-7. If the average atomic mass of lithium is 6.941 amu, which isotope is the most abundant? How do you know? (No calculation necessary) 3. Iodine is 80 % 127I, 17 % 126I, and 3 % 128I. Calculate the average atomic mass of iodine. 4. In a sample of 120 atoms of hafnium, 10 have a mass of 176 amu, 24 have a mass of 177 amu, 32 have a mass of 178 amu, 14 have a mass of 179 amu, and 40 have a mass of 180. amu? a. Calculate the relative abundance of each isotope of hafnium. % Hf-176 % Hf-177 b. Calculate the average atomic mass of hafnium. % Hf-178 % Hf-179 % Hf-180 5. Calculate the average atomic mass of bromine. The two isotopes of bromine have atomic masses and relative abundances of 78.92 amu (50.69 %) and 80.92 amu (49.31 %). 6. Calculate the average atomic mass of iridium using the following data for two iridium isotopes. Isotope mass (amu) relative abundance (%) Ir-191 191.0 37.58 Ir-193 193.0 62.42 7. Rubidium is a soft, silvery-white metal that has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2 % and the abundance of 87Rb is 27.8 %, what is the average atomic mass of rubidium? 8. Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. If the abundance of 234U is 0.01 %, the abundance of 235U is 0.71 %, and the abundance of 238U is 99.28 %, what is the average atomic mass of uranium? 9. Titanium has five common isotopes: 46Ti (8.0 %), 47Ti (7.8 %), 48Ti (73.4 %), 49Ti (5.5 %), 50Ti (5.3 %). How many atoms of each isotope would be in a sample of 2300 titanium atoms? Ti-46 Ti-47 Ti-48 Ti-49 Ti-50