Average Atomic Mass Worksheet
advertisement
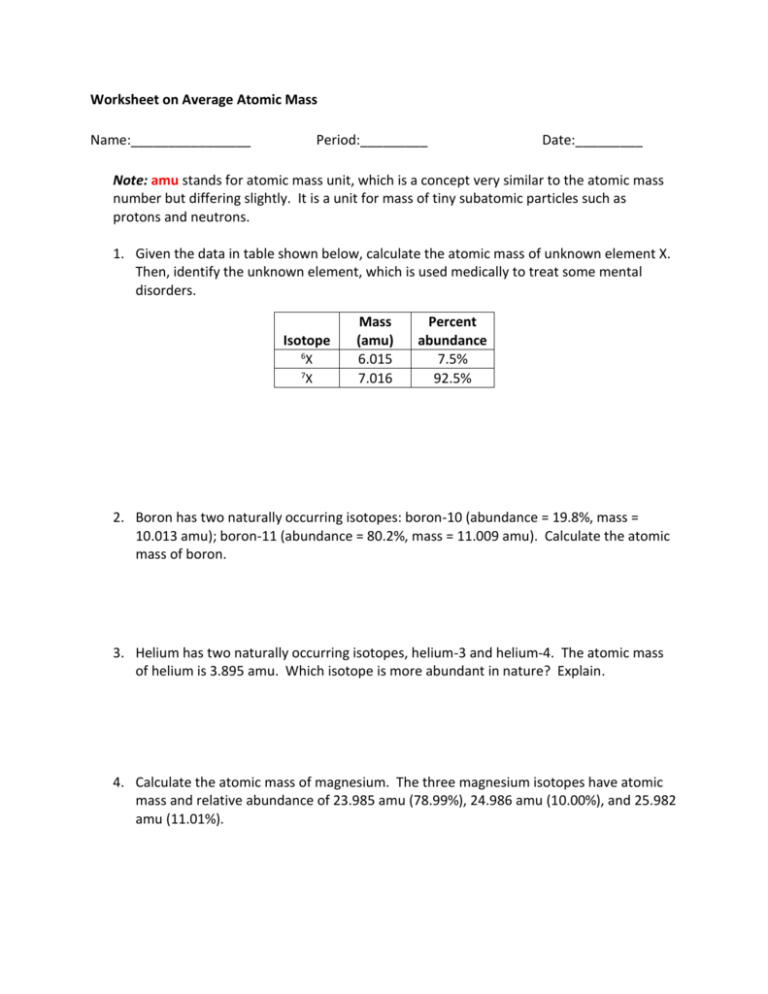
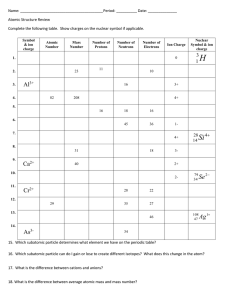
Worksheet on Average Atomic Mass Name:________________ Period:_________ Date:_________ Note: amu stands for atomic mass unit, which is a concept very similar to the atomic mass number but differing slightly. It is a unit for mass of tiny subatomic particles such as protons and neutrons. 1. Given the data in table shown below, calculate the atomic mass of unknown element X. Then, identify the unknown element, which is used medically to treat some mental disorders. Isotope 6 X X 7 Mass (amu) 6.015 7.016 Percent abundance 7.5% 92.5% 2. Boron has two naturally occurring isotopes: boron-10 (abundance = 19.8%, mass = 10.013 amu); boron-11 (abundance = 80.2%, mass = 11.009 amu). Calculate the atomic mass of boron. 3. Helium has two naturally occurring isotopes, helium-3 and helium-4. The atomic mass of helium is 3.895 amu. Which isotope is more abundant in nature? Explain. 4. Calculate the atomic mass of magnesium. The three magnesium isotopes have atomic mass and relative abundance of 23.985 amu (78.99%), 24.986 amu (10.00%), and 25.982 amu (11.01%).