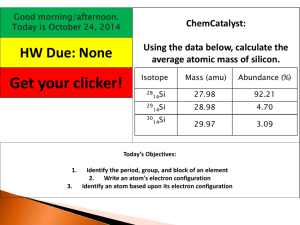
Calculating Atomic Mass
advertisement
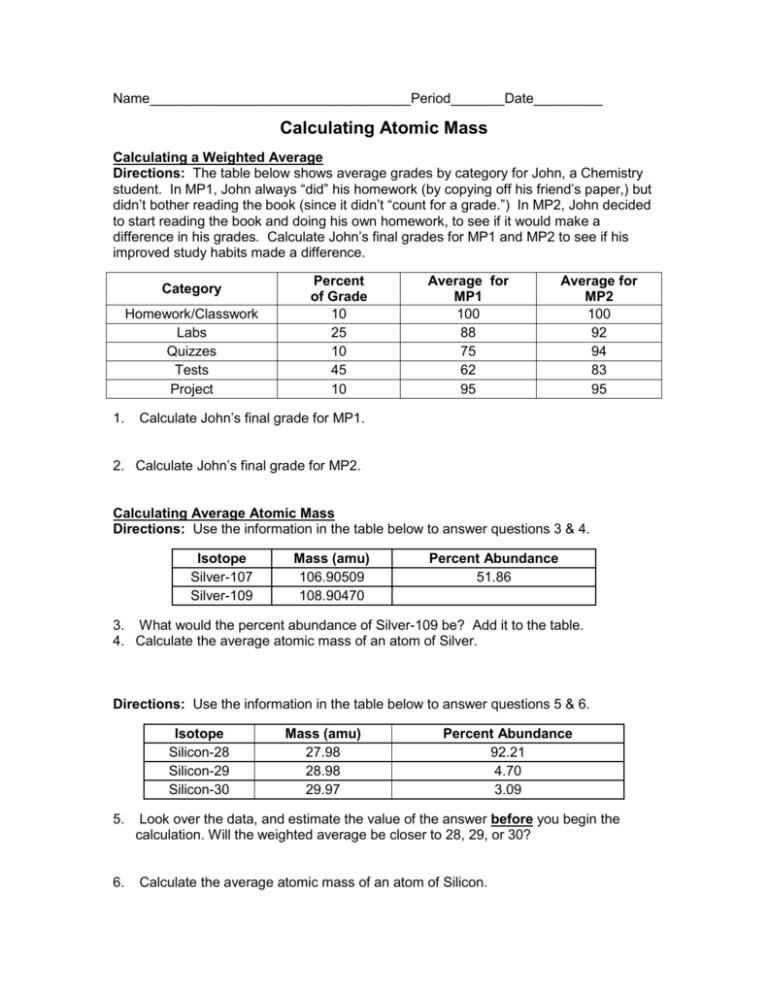
Name__________________________________Period_______Date_________ Calculating Atomic Mass Calculating a Weighted Average Directions: The table below shows average grades by category for John, a Chemistry student. In MP1, John always “did” his homework (by copying off his friend’s paper,) but didn’t bother reading the book (since it didn’t “count for a grade.”) In MP2, John decided to start reading the book and doing his own homework, to see if it would make a difference in his grades. Calculate John’s final grades for MP1 and MP2 to see if his improved study habits made a difference. Category Homework/Classwork Labs Quizzes Tests Project 1. Percent of Grade 10 25 10 45 10 Average for MP1 100 88 75 62 95 Average for MP2 100 92 94 83 95 Calculate John’s final grade for MP1. 2. Calculate John’s final grade for MP2. Calculating Average Atomic Mass Directions: Use the information in the table below to answer questions 3 & 4. Isotope Silver-107 Silver-109 Mass (amu) 106.90509 108.90470 Percent Abundance 51.86 3. What would the percent abundance of Silver-109 be? Add it to the table. 4. Calculate the average atomic mass of an atom of Silver. Directions: Use the information in the table below to answer questions 5 & 6. Isotope Silicon-28 Silicon-29 Silicon-30 Mass (amu) 27.98 28.98 29.97 Percent Abundance 92.21 4.70 3.09 5. Look over the data, and estimate the value of the answer before you begin the calculation. Will the weighted average be closer to 28, 29, or 30? 6. Calculate the average atomic mass of an atom of Silicon. Directions: Use the information in the table below to answer questions 7 & 8. Isotope name Iron-54 Iron-56 Iron-57 Iron-58 Percent Abundance 5.90 91.72 2.10 0.280 7. Estimate the average atomic mass of Iron. 8. Calculate the average atomic mass of Iron. Mass (amu) 53.94 55.93 56.94 57.93 Directions: Samples of an unknown element X were collected and their masses were recorded. Use the information presented in the data table to answer questions 9-13. Isotope Mass (amu) 1 37.765 Percent Abundance 9.67 2 39.056 78.68 3 40.003 11.34 4 41.060 0.31 Mass Number 9. Fill in the mass number for each sample of element X in the data table. 10. What is the most common isotope of element X? 11. Calculate the average atomic mass of element X. 12. Using your periodic table, identify element X based on its average atomic mass. What is the name of this element? 13. What is the atomic number of this element?