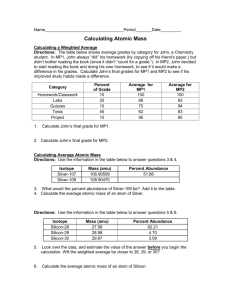
Isotopes
advertisement

Isotopes Show your work 1. How many protons, electrons, and neutrons make up an atom of Protons: _________ Electrons: _________ Neutrons: _________ bromine-80? 2. Calculate the average atomic mass of boron from the following information: Isotope % Abundance Atomic Mass 10 B 18.8 10.0 11 B 81.2 11.0 Average atomic mass: _________________ 3. Calculate the average atomic mass of element X in which isotope one (42.00%) weighs 138.2 amu and its second isotope (58.00%) weighs 151.6 amu. Average atomic mass:__________________ 4. Calculate the average atomic mass of element Z in which the first isotope found in 28.5% abundance weighs 200.0 amu and the other isotope weighs 220.0 amu. Average atomic mass: __________________ 5. Antimony has two common isotopes. If one of the isotopes is antimony-121 with an atomic mass of 120.9038 amu and an abundance of 57.25%, what is the atomic mass (to 4 significant figures) of the second isotope? The average atomic mass of antimony is 121.75. Atomic mass of second isotope: ___________