Chemistry Worksheet: Atoms, Radioactivity, Trends
advertisement
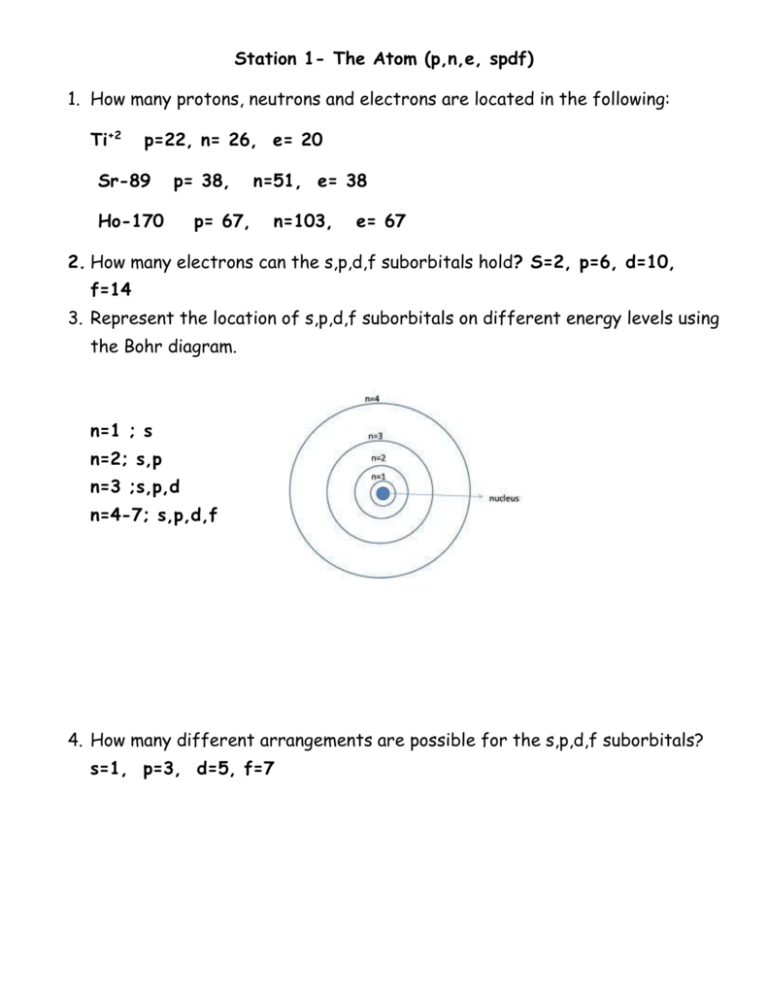
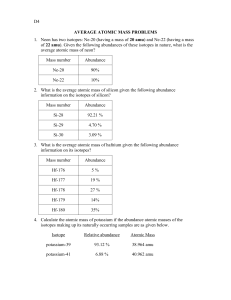
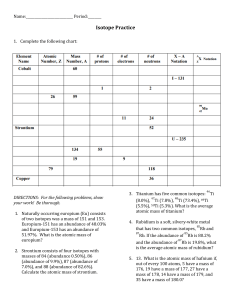
Station 1- The Atom (p,n,e, spdf) 1. How many protons, neutrons and electrons are located in the following: Ti+2 p=22, n= 26, e= 20 Sr-89 p= 38, Ho-170 p= 67, n=51, e= 38 n=103, e= 67 2. How many electrons can the s,p,d,f suborbitals hold? S=2, p=6, d=10, f=14 3. Represent the location of s,p,d,f suborbitals on different energy levels using the Bohr diagram. n=1 ; s n=2; s,p n=3 ;s,p,d n=4-7; s,p,d,f 4. How many different arrangements are possible for the s,p,d,f suborbitals? s=1, p=3, d=5, f=7 Station 2- Average Atomic Mass 1) Rubidium has two common isotopes, Rb-85 and Rb-87. If the abundance of Rb85 is 72.2% and the abundance of Rb-87 is 27.8%, what is the average atomic mass of rubidium? 85.56 amu 2) Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of 235U is 0.71%, and the abundance of 238 U is 99.28%, what is the average atomic mass of uranium? 237.98 amu 3) Titanium has five common isotopes: Ti-46 (8.0%), Ti-47 (7.8%), Ti-48 (73.4%), Ti-49 (5.5%), Ti-50 (5.3%). What is the average atomic mass of titanium? 47.92 amu 4) There are four isotopes of lead. Data on their atomic structure can be found in the table. Find the average atomic mass of lead in the space below. 207.70 amu Isotope A B C D Protons 82 82 82 82 Neutrons 122 124 125 126 Percent Abundance 1.37% 26.26% 20.82% 51.55% Station 3- Radioactivity Including Equations, Fission and Fusion 1. Write the equation for the alpha and beta decay for the following isotopes: Bi-210, Tb-155 and Md-260 2. What is produced during fission and fusion? Fission- 2 stable atoms, 3 neutrons and a lot of energy Fusion- one stable neutron and a lot of energy 3. Write the fission reaction for Po-211 if Xe-133, 3 particles and another nucleus are formed. 4. What elements are involved in the reaction of the sun? Two hydrogen atoms are combined to form 1 helium atom. 5. The radioactive decay of uranium-238 ends with lead-206 and involves many steps. The first four steps are alpha, beta, beta, alpha. Write the first four equations for the decay of uranium-238 to lead-206. Station 4- 1/2 Life Problems 1. If the original mass of Ra-226 is 20.0 grams and the half-life is 1600 years, how much remains after 9600 years? 0.3125 g Ra-226 2. If 14 grams of thorium-234 remains after 120 days, determine the mass of the original sample. The half-life of thorium-234 is 24 days. 448 g thorium-234 3. What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 grams in 21.6 seconds? 7.2sec Station 5- Electron Configuration, Dot and Box 1. Write the electron box diagrams for Aluminum, Zinc and Rubidium. 2. Write the short hand (or noble gas) configuration for the three elements above. Al: [Ne] 3s23p1 Zinc: [Ar] 4s2 3d10 Rubidium: [Kr] 5s1 3. Draw the electron dot for Astatine, Germanium and Francium. 4. Give the name, symbol and number of valence electrons for each of the following elements: a. c. 1s22s22p63s1 b. Sodium – 1 VE Nitrogen – 5 VE 1s22s22p63s23p63d34s2 d. Vandium- 2 VE 1s22s22p3 1s22s22p63s23p64s1 Potassium – 1VE Station 6- Trends 1. In each of the following pairs, circle the species with the higher ionization energy: (a) Li or Cs (b) Cl or I (c) Ca or Br (d) Li or Ne (e) B or Al 2. In each of the following pairs, circle the species with the larger atomic radius: (a) Mg or Ba (b) S or S2- (c) Cu+2 or Cu (d) He or H- (e) Na or Cl 3. In each of the following pairs, circle the species with the lower electronegativity energy: (a) Mg or Ba (b) P or N (c) Ca or Br (d) Na or Ar (e) C or Ge Station 7- Waves 1. Are energy and wavelength inversely or directly proportional? Inversely proportional 2. The higher the frequency, the __higher__ (higher or lower) the energy. The higher the wavelength, the (higher or lower) the energy. 3. Which color has the longest wavelength? Red 4. Which color has the highest frequency? Violet 5. If an electron moves from energy level 5 to energy level 2 which of the following is true: 1. Its wavelength is 486 x 10-9. False – 434 x 10-9 m 2. The energy released as the electron moves will produce a green light. False - Blue 3. It has more energy than violet light. False- Less energy 4. Its frequency is greater than the frequency of microwaves. True