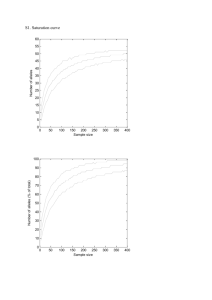
Supplementary material - Springer Static Content Server
advertisement

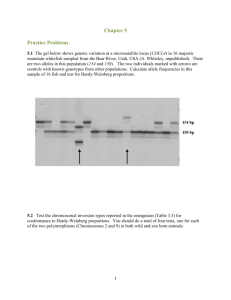
Supplementary material Microsatellite testing Methods Deviations from Hardy-Weinberg could theoretically result from the presence of null alleles, deviations from the Hardy-Weinberg assumption of random mating (linkage disequilibrium), sampling from multiple populations, or sampling siblings and parent/offspring pairs. Because null alleles or cryptic genetic divisions would violate our inherent assumptions and potentially bias our interpretations, all of these possibilities were tested for in our analyses as follows. We tested for the presence of null alleles within the program Micro-checker (Van Oosterhout et al. 2004), and looked for linkage disequilibrium among loci using Genepop version 4.0. To ensure we had sampled from a single population (as the within-population scale is the focus of this study), we used the program Structure version 2.2 (Pritchard et al. 2000). In this analysis we checked for genotype clusters ignoring geographic locations, by maximising Hardy-Weinberg equilibrium and minimising linkage disequlibria among groups of individuals. We ran Structure using admixture of ancestry and the correlated allele frequencies model (recommended for detecting subtle population structure; Pritchard et al. 2007), and tested whether individuals could be assigned to between one and ten populations (K). For each estimate of K, we carried out 10 independent runs. We assessed relatedness between sampled individuals using a parentage analysis (maternal and paternal without known parents) implemented in the software program CERVUS version 3.0 (Kalinowski et al. 2007). The process first involves simulating a new set of individuals based on the observed allele frequencies in the real data (in this case we set the number of simulations was 10000). From this new data set offspring are ‘produced’ using Mendelian principles. The geneotypes observed among these individuals are then used to create a likelihood distribution (based on LOD or Delta values, see Kalinowski et al. (2007) for details) of relatedness within a population. Based on this distribution, parentage or relatedness between two observed individuals can be assessed, and assigned a probability value. To ensure that loci that deviated from Hardy-Weinberg equilibrium did not have a skewing effect on the results, we also carried out the Mantel test analyses (as described in the main text) between ar (calculated only from loci within Hardy-Weinberg equilibrium) and each of the models for all individuals for both white-browed and yellow-throated scrubwren. Results No null alleles were detected (at p < 0.05), and all loci had at least one allele evident for every individual. A single statistically significant instance of linkage disequilibrium was found for each species; for the white-browed scrubwren between AAGG-114 and AACC-1 (p = 0.04 and p = 0.05 respectively), and for the yellow-throated scrubwren between AAGG-63 and AACC-33 (p = 0.03 and p = 0.04 respectively), though none remained significant following a Bonferroni correction for multiple tests. We were unable to assign populations using the program Structure at K = 2 or greater within the program, and K = 1 had the greatest likelihood. This confirmed we had probably sampled from a single population of both species. The parentage analysis revealed that for both species, the probability that any pair of individuals in this study were sibling/half-sibling or parent/off-spring was <0.0001. This suggests that direct co-ancestry between sampled individuals is not a confounding factor in this study. The results, when calculated without loci that deviated from Hardy-Weinberg, produced an identical ranking order as when all loci were used. Furthermore, as there was no evidence of close relatives or null alleles in the data set we present results using all loci from Table S1. The results of the Mantel tests using only microsatellite loci that were in HardyWeinberg equilibrium are presented in Table S2. The ranking order of the models is very similar to that calculated with all the microsatellite data (with the top models staying the same), so we have presented the full data set in the main text. Table S1. Number of alleles per locus, expected and observed heterozygosity and the significance of deviations from Hardy-Weinberg equilibrium calculated for each locus and globally for samples of yellow-throated and white-browed scrubwrens. Loci for which there was a significant heterozygote deficiency following sequential Bonferroni correction are marked with an asterix *. White-browed scrubwren Locus AACC-90 AACC-83 AAGG-63 AACC-33 AAGG-74 AAGG-75 AAGG-114 AACC-1 All loci Yellow-throated scrubwren Number of alleles Expected heterozygosity Observed heterozygosity P-value 6 12 12 8 0.30 0.88 0.80 0.82 0.31 0.95 0.71 0.36 0.31 0.951 0.020 0.001* 25 14 8 0.49 0.78 0.77 0.76 0.17 0.79 0.6 0.59 0.11 0.99 0.001* 0.001 Number of alleles 3 2 6 7 15 33 Expected heterozygosity Observed heterozygosity P-value 0.47 0.20 0.73 0.79 0.90 0.47 0.51 0.17 0.78 0.51 0.59 0.08 0.417 0.419 0.312 0.001* 0.005 0.004 0.59 0.44 0.001 Table S2. Results from Mantel tests for each model (see Table 2 in the main document) tested against individual based genetic distances (ar) for both the white-browed scrubwren and the yellow-throated scrubwren from all individuals calculated from microsatellite data without the loci that deviated from Hardy-Weinberg equilibrium (see Table S1). Ranking is based on the Mantel r correlation coefficient and p-value. Results that were significant following Bonferroni correction for multiple tests are indicated with an asterix *. White-browed scrubwren (generalist species) Sub-model label Overall rank Mantel r correlation coefficient P- value Model 1 1 0.121 0.003* Model 2a 14 0.067 0.10 Model 2b 13 0.068 0.05 Model 3a 2 0.079 0.08 Model 3b 8 0.075 0.04 Model 4a 5 0.078 0.23 Model 4b 11 0.071 0.05 Model 4c 3 0.078 0.45 Model 4d 10 0.071 0.03 Model 5a 12 0.07 0.05 Model 5b 9 0.075 0.06 Model 5c 4 0.078 0.42 Model 5d 16 0.005 0.76 Model 5e 15 0.002 0.93 Model 5f 17 0.001 0.31 Model 5g 7 0.076 0.12 Model 5h 6 0.076 0.05 Yellow-throated scrubwren (specialist species) Sub-model label Overall rank Mantel r correlation coefficient P- value Model 1 14 0.075 0.28 Model 2a 16 0.071 0.46 Model 2b 15 0.071 0.51 Model 3a 8 0.092 0.1 Model 3b 7 0.093 0.03 Model 4a 1 0.131 0.001* Model 4b 9 0.09 0.07 Model 4c 2 0.11 0.06 Model 4d 11 0.085 0.09 Model 5a 3 0.105 0.03 Model 5b 5 0.099 0.061 Model 5c 17 0.054 0.14 Model 5d 10 0.09 0.09 Model 5e 4 0.099 0.09 Model 5f 12 0.081 0.18 Model 5g 13 0.081 0.26 Model 5h 6 0.099 0.05 REFERENCES Kalinowski S.T., Taper M.L. and Marshall T.C. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology 16: 1099-1006. Pritchard J.K., Stephens M. and Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945-959. Pritchard J.K., Wen X. and Falush D. 2007. Documentation for structure software: Version 2.2. University of Chicago. Van Oosterhout C., Hutchinson W.F., Wills D.P.M. and Shipley P. 2004. MICROCHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes 4: 535-538.