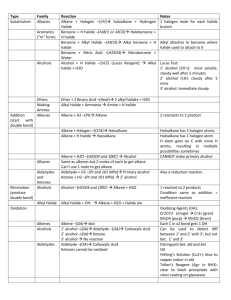
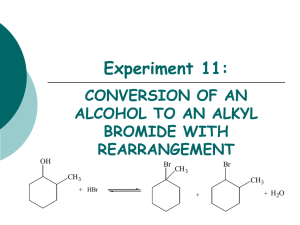
Class XII Chemistry
advertisement
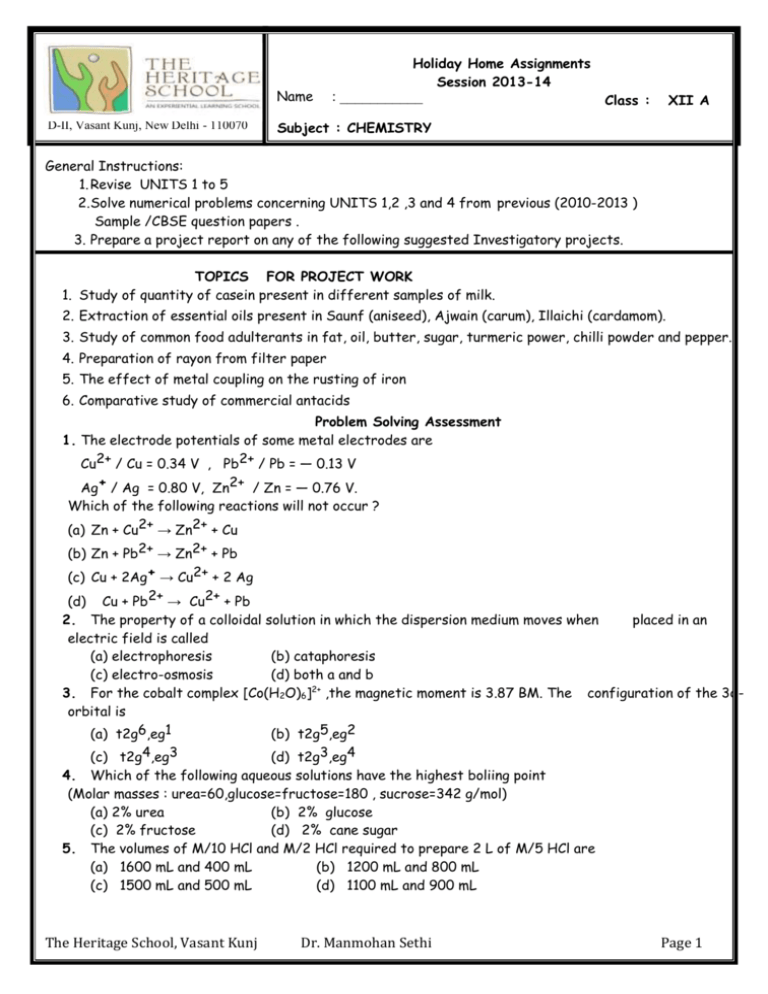
Name D-II, Vasant Kunj, New Delhi - 110070 Holiday Home Assignments Session 2013-14 : ___________ Class : XII A Subject : CHEMISTRY General Instructions: 1. Revise UNITS 1 to 5 2.Solve numerical problems concerning UNITS 1,2 ,3 and 4 from previous (2010-2013 ) Sample /CBSE question papers . 3. Prepare a project report on any of the following suggested Investigatory projects. TOPICS FOR PROJECT WORK 1. Study of quantity of casein present in different samples of milk. 2. Extraction of essential oils present in Saunf (aniseed), Ajwain (carum), Illaichi (cardamom). 3. Study of common food adulterants in fat, oil, butter, sugar, turmeric power, chilli powder and pepper. 4. Preparation of rayon from filter paper 5. The effect of metal coupling on the rusting of iron 6. Comparative study of commercial antacids Problem Solving Assessment 1. The electrode potentials of some metal electrodes are Cu2+ / Cu = 0.34 V , Pb2+ / Pb = — 0.13 V Ag+ / Ag = 0.80 V, Zn2+ / Zn = — 0.76 V. Which of the following reactions will not occur ? (a) Zn + Cu2+ → Zn2+ + Cu (b) Zn + Pb2+ → Zn2+ + Pb (c) Cu + 2Ag+ → Cu2+ + 2 Ag (d) Cu + Pb2+ → Cu2+ + Pb 2. The property of a colloidal solution in which the dispersion medium moves when placed in an electric field is called (a) electrophoresis (b) cataphoresis (c) electro-osmosis (d) both a and b 3. For the cobalt complex [Co(H2O)6]2+ ,the magnetic moment is 3.87 BM. The configuration of the 3dorbital is (a) t2g6,eg1 (b) t2g5,eg2 (c) t2g4,eg3 (d) t2g3,eg4 4. Which of the following aqueous solutions have the highest boliing point (Molar masses : urea=60,glucose=fructose=180 , sucrose=342 g/mol) (a) 2% urea (b) 2% glucose (c) 2% fructose (d) 2% cane sugar 5. The volumes of M/10 HCl and M/2 HCl required to prepare 2 L of M/5 HCl are (a) 1600 mL and 400 mL (b) 1200 mL and 800 mL (c) 1500 mL and 500 mL (d) 1100 mL and 900 mL The Heritage School, Vasant Kunj Dr. Manmohan Sethi Page 1 6. An X molal solution of a compound in benzene has a mole fraction of solute equal to 0.2.The value of X is (a) 1.4 (b) 14 (c) 3.2 (d) 32 7. A compound AB crystallizes in a BCC lattice with anions B at all the corners and cation A in the body of the cube. The body diagonal is found to be 716 pm. If the radius of the cation A is 170, the radius of the anion is (a) 376 pm (b) 94 pm (c) 188 pm (d) 179 pm 8. Which of the following expressions is correct for first order reaction? [CO] refers to initial concentration of reactant. (a) t½ [CO]o (b) t½ [CO]–1 (c) t½ [CO]–2 (d) t½ [CO] 9. If the observed and normal osmotic pressures of KCl solution at 25°C are 5.76 and 3.2 atms. What will be its percentage ionization at this temperature? (a) 100 (b) 28 (b) 80 (c) 67 10. For the reaction 2 NO (g) + O2 (g) → 2 NO2 (g) volume is suddenly reduced to half its original value by increasing the pressure on it. If the reaction is first order with respect to O2 and second order with respect to NO , the rate of reaction will (a) Increase to four times its initial value (b) diminish to one-fourth its original value (c) Increase to eight times its initial value (d) diminish to one-eighth its original value 11. Absolute alcohol cannot be prepared from rectified spirit by simple distillation because (a) the boiling points of water and alcohol are very close (b) water and alcohol form a constant boiling mixture (azeotrope) (c) C2H5OH form H-bond with water (d) water and alcohol form a non-ideal solution 12. The elimination of HX from an alkyl halide forms an alkene. The order of the elimination reactions is (a) 3° halide > 2° halide > 1° halide (c) 1° halide = 2° halide > 3° halide (b) 1° halide > 2° halide > 3° halide (d) 2° halide > 1° halide > 3° halide 13. Which of the following compounds on warming with iodine solution and NaOH will not give iodoform ? (a) CH3CH2OH (c) (CH3)2CHOH (b) CH3COCH3 (d) CH3OH 14. Which organic compound among the following is expected to undergo nitration more easily and readily to form the corresponding nitro derivative employing the usual nitrating mixture (a) C6H6 (b) C6H5CH3 (c) C6H5NO2 The Heritage School, Vasant Kunj (d) C6H4CCl3 Dr. Manmohan Sethi Page 2 15. Optical isomerism is shown by (a) 1- Butanol (b) (c) 2- Butanol (d) The Heritage School, Vasant Kunj 2-methylpropan-1-ol 2-methylpropan-2-ol Dr. Manmohan Sethi Page 3