Halogeno
advertisement

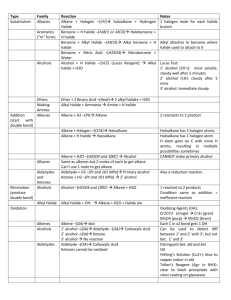
Halogeno-compounds 1. Classification: (a) Primary halide (1 halide) H H H H H H H H H Cl (b) Secondary halide (2 halide) Cl H H H H H H H H H (c) Tertiary halide (3 halide) H H H H Cl H H H H H 2. Preparation (a) Alcohol reacts with hydrogen halide R OH HCl ZnCl 2 R Cl H 2 O (Lucas test) The above reaction is a SN1 reaction, and ZnCl2 is used as a catalyst. The rate follows the following order. 3 > 2 > 1 For bromide and iodide, they are more reactive, and catalyst is not required. NaBr H 3 PO4 NaH 2 PO4 HBr Phosphoric acid is a non-volatile acid. R OH HBr R Br H 2 O NaI H 3 PO4 NaH 2 PO4 HI R OH HI R I H 2 O Iodide > bromide > chloride (b) Alcohol reacts with phosphorus trichloride 3ROH PX 3 3RX H 3 PO3 (c) Alcohol reacts with phosphorus pentachloride R O H PX 3 R X POX 3 HX (white fumes, a test for –OH group) (d) Alcohol reacts with thionyl chloride R O H SOCl 2 R Cl SO2 HCl 3. Properties 2 types of nucleophilic substitution (a) Unimolecular nucleophilic substitution / SN1: Halide / page 1 Br OH H + O H + H Br Reaction mechanism: Step I H H Br H H H H H H rds H H H H H H + H H H + Br - H * carbonium ion is trigonal planar (sp2) Step II H + H OH O + + + fast fast H + H H O * racemic mixture if the halide is optically active rate = k[halide] rate order = 1 Enthalpy carbonium ion alcohol halide + water Reaction Profile (b) Bimolecular nucleophilic substitution / SN2: Br + OH - NaOH(aq) OH + Br - Reaction mechanism: Rate determining step Halide / page 2 H H H + Br H OH H - H H H Br Br - + OH H OH H * The transition state is a trigonal bipyramid. d-isomer l-isomer The reaction causes optical inversion. rate = k[halide][nucleophile] rate order = 2 Transition state Enthalpy alcohol halide + OH Reaction Profile (c) Competition between SN1 and SN2: (i) Structure of the halide: (I) Electronic effect Inductive effect: Highly branched halide can form stable carbonium ion, so it prefers SN1. Resonance effect: Conjugated bond can form stable carbonium ion, so it prefers SN1. (II) Steric hindrance Highly branched halide prefers SN1, because the intermediate becomes less bulky. Primary halide prefers SN2. (ii) Nucleophilic strength: Strong nucleophile makes SN2 easily. (iii) Polarity of solvent: Polar solvent can stabilize the carbonium ion. Rate of SN1: 3 > 2 > 1 Rate of SN2: 1 > 2 > 3 Overall rate: 3 > 1 > 2 (halide) (halide) Halide / page 3 rate 1 2 3 (d) Other examples of nucleophilic substitution: (i) Halides reacts with cyanide ion (KCN, NaCN in alcohol solution) N Cl O + H / H O 2 NaCN(alcohol) OH (ii) Amine formation H H NH H 3 H NH2 H H Br CH3 N CH Br 3 H CH Br 3 CH3 1 amine CH3 N CH Br 3 Br CH3 CH3 +N CH3 CHCH 3 3 CH3 2 amine - 3 amine tetramethylammonium bromide (e) Elimination Cl CH3 CH3 CH3 H CH3 + OH CH3 CH3 - + CH3 H O H + Cl - CH3 The driving force of elimination if steric hindrance. Factors affecting elimination: (i) highly branched halide: The product becomes less bulky. (ii) non-aqueous solution (non-polar solvent): The equilibrium shifts to the RHS. (iii)high temperature: The equilibrium shifts to the RHS, because alkene has a lower bp. (iv) strong base: It removes hydrogen ion, and forces the equilibrium to RHS. Conclusion: Halide SN1 SN2 E 1 strong nucleophile room temperature halide with bulky group strong base high temperature non-aqueous solution 2 low temperature aqueous solution weak base low temperature strong nucleophile high temperature non-aqueous solution strong base 3 low temperature aqueous solution weak base high temperature non-aqueous solution strong base Halide / page 4