Alkane Reactions
advertisement

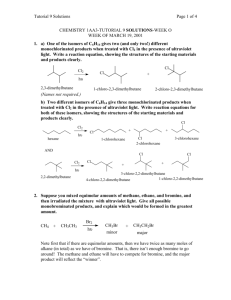
Alkane Reactions t • Alkanes have only single, covalent, nonpolar bonds • Alkanes are relatively inert to most chemical reagents • Alkanes are also called paraffins (“little affinity”) 1. Combustion Reaction • also called destructive oxidation • When used as a fuel, such as natural gas; gasoline • Products of combustion are always carbon dioxide and water • Ex.; C3H8 + 5O2 3CO2 + 4H20 2. Substitution Reactions • a hydrogen is removed and another species replaces it • a. Halogenation - a substitution of a hydrogen with a halogen (either Cl or Br) - this is sometimes called chlorination or bromination • Ex.; Chlorinate methane CH4 Cl2 CH3Cl + HCl heat or sunlight • Halogenation does not occur in one step; it involves a series of steps called the Free Radical Chain Mechanism Step 1: Chain Initiation – this step provides the chlorine atoms that get this going. Chlorine molecule absorbs energy and splits into free radical chlorines. A free radical is an atom or group possessing an odd (unpaired) electron. Free radicals are extremely reactive. Cl:Cl Heat or sunlight 2Cl· • Step 2: Chain Propogation - this step is where almost all the reactant is consumed and all the products are formed. The chlorine free radical attacks the hydrocarbon and removes a hydrogen, leaving a methyl free radical. The methyl free radical collides with a chlorine molecule and pulls off a chlorine. • Step 3: Chain Termination - if any two radicals combine, chains are terminated. There are three possibilities: • Successive substitutions are possible. You can dichlorinate, trichlorinate, tetrachlorinate…until there are no hydrogens left to be replaced. • CH4 + Cl2/uv CH3Cl + Cl2/uv CH2Cl2 + Cl2/uv CHCl3 + Cl2/uv CCl4 • Larger alkanes can produce side reactions and by-products • Ex.: Dichlorinate ethane • CH3—CH3 Cl2 CH3—CH2Cl uv • CH3—CH2Cl Cl2 uv CH3-CHCl2 + CH2Cl-CH2Cl dichloroethane 1,2-dichloroethane • b. Nitration - substitution of an –NO2 group. Only mononitration occurs. – Reagent is nitric acid (HNO3) – Condition for reaction to proceed: 400°C • In higher alkanes, breaking of carboncarbon bonds as well as carbon-hydrogen bonds occurs • Ex.; Nitrate methane • Ex.; Nitrate propane Synthesis of Alkanes • 1. Wurtz Reaction – treatment of an alkyl halide with metallic sodium • Product is always symmetrical and has twice as many carbons as the original alkyl halide • Ex.; React bromoethane with sodium • Ex.; React 2-bromopropane with sodium • 2. Reduction of an Alkyl Halide replacement of a halide with hydrogen using zinc and acetic acid • Ex.; React bromoethane with zinc & acetic acid • Ex.; React 2-bromopropane with zinc and acetic acid • 3. Hydrolysis of a Grignard Reagent - a Grignard reagent is an organomagnesium halide • Grignard reagents are prepared from an alkyle halide that reacts with magnesium in an ether solution. When the Grignard is in the presence of water it acts as a strong base. • Ex.; React bromopropane with Mg/ether in water. • 4. Catalytic Hydrogenation - addition of hydrogen across a double bond • Uses either Pt or Pd as the catalyst • Ex.; Hydrogenate ethene • Ex.; Hydrogenate propene