Electrorefining
advertisement

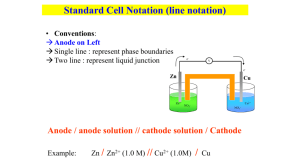
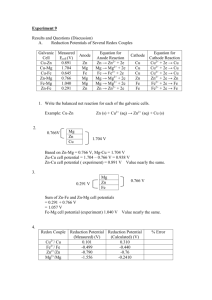
Do you wanna be rich? We can help you! Let's see a video first which teaches you the way to be rich! Electrorefining Phyllis Zhang Diana Gao Cindy Zhang Electrorefining is the process of purifying a metal by electrolysis. - Pure Cu (Cathode) DC power supply + CuSO4(aq) Slab of impure copper containing such impurities as: Zn, Pb, Ag(Anode) Examine the following cell - DC power supply Zn2+ Cu2+ Pure Cu (Cathode) + Pb2+ Cu2+ CuSO4(aq) Cu2+ Cu2+ Cu2+ Zn2+ 2+ Cu Cu2+ Cu, Zn, Pb, Ag The cathode reaction: The Cu2+ in solution is preferentially reduced at the cathode but none of the Pb2+ or Zn2+ can be reduced.(Cu2+ exists in larger amounts and has a higher reduction potential than Zn2+ and Pb2+.) In summary: No metals above Cu on the table can oxidize and go into solution, and no metal below Cu on the chart can reduce and come back out of solution. As a result, only Cu is involved in both the oxidation and reduction. Impure anode pure cathode Exercise----P246 An aqueous solution of SnSO4 was electrolyzed using tin electrodes. The anode contained a few percent of silver, copper and zinc as impurities. Which substances(s) will be oxidized at the anode? Which substances(s) will be reduced at the cathode? Applications lead copper gold silver zinc aluminium chromium cobalt manganese the rare-earth and alkali metals Bibliography: the video: http://v.youku.com/v_show/id_XMjk5MDEwMTM2.html the pictures: http://image.baidu.com information: http://en.wikipedia.org/wiki/Electrorefining That's all! Thank you! Zn Pt Cu Ag Pb Au