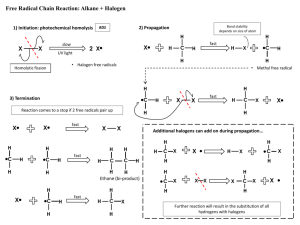
Summary of SN1/SN2 and E1/E2 Chemistry
advertisement

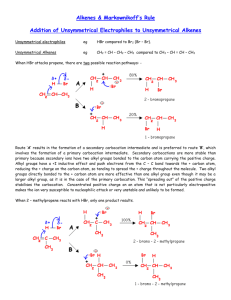
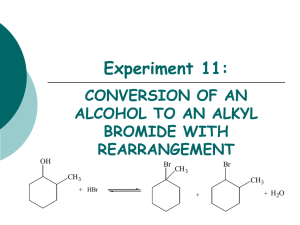
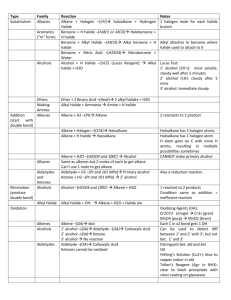
Summary of SN1/SN2 and E1/E2 Chemistry SN2 Reactions: 1. Rate depends upon concentration of the alkyl halide and the concentration of the nucleophile. 2. As the carbon bearing the halide becomes more substituted, the rate of reaction decreases. 3. The rate of reaction also decreases as the size of a substituent on the carbon bearing the halide increases. 4. Substitution of a halide attached to a chirality center leads to the formation of only one stereoisomer in which the configuration of the product is inverted relative to the starting configuration of the alkyl halide. 5. The SN2 reaction is a one-step bimolecular process with one transition state. Leaving group ability increases with decreasing basicity. Nucleophilicity increases with increasing basicity in polar aprotic solvents. Nucleophilicity typically increases with increasing atomic size in polar protic solvents. In protic solvents, nucleophilicity increases with decreasing basicity. Nucleohpilicity decreases with increasing steric bulk of the nucleophile. SN2 reversibility is dependent upon the difference in basicity (compare pKa’s of the conjugate acids) of the nucleophile and leaving group: little difference reversible large difference irreversible SN1 Reactions: 1. Rate depends upon only the concentration of the alkyl halide. It is independent of nucleophile concentration. 2. As the carbon bearing the halide becomes more substituted, the rate of reaction increases. This trend parallels carbocation stability. 3. Substitution of a halide attached to a chirality center leads to the formation of a racemic mixture. 4. The SN1 reaction is a two-step unimolecular process with one intermediate and two transition states. Leaving group ability increases with decreasing basicity. The addition of the nucleophile to the carbocation intermediate is not the rate limiting step (RLS). The RLS is the formation of the carbocation intermediate. Therefore, nucleophile reactivity has no effect on the S N1 reaction. The solvent is the nucleophile in many SN1 reactions. This is called a solvolysis reaction. 1,2-Hydride shifts and 1,2-methyl shifts will occur in SN1 reactions if the rearrangement leads to a more stable carbocation. These rearrangements do not occur for obvious reasons in the S N2 reaction. E2 Reactions: 1. Rate depends upon the concentration of the alkyl halide and the base. 2. The E2 reaction is a one-step bimolecular process with one transition state. 3. The E2 reaction is regioselective because when two different elimination products are possible from the same starting alkyl halide, the product with the more substituted double bond typically predominates (Zaitsev’s rule). Exceptions to Zaitsev’s rule: (a) As the steric bulk of the base increases, the less substituted product alkene becomes more favored. (b) Dehydrohalogenation of alkyl fluorides results in the less substituted alkene predominating. The increased stength of the C-F bond results in quite a build-up of electron density on the -carbon. Therefore, transition state stability parallels carbanion stability. (c) Finally, Zaitsev’s rule is not observed when the less substituted product results in a conjugated diene, or when the resulting double bond of the less substituted alkene is conjugated with a benzene ring. E1 Reactions: 1. Rate depends only upon the concentration of the alkyl halide. 2. The E1 reaction is a two-step unimolecular process with one intermediate and two transition states. 3. Product distribution may be predicted using Zaitsev’s rule. In other words, the more substituted alkene ususally predominates. 4. Since the rate limiting step is the formation of the carbocation, then reactivity parallels carbocation stability. 1,2-Hydride shifts and 1,2-methyl shifts will occur in E1 reactions if the rearrangement leads to a more stable carbocation. These rearrangements do not occur for obvious reasons in the E2 reaction.