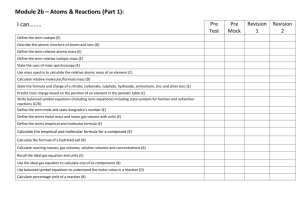
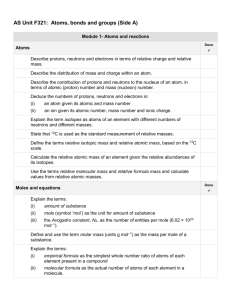
AS Chemistry topic checklist

AS Chemistry – Unit 2
REDOX REACTIONS AND GROUP 7
Be able to assign oxidation states, and use these to work out formulae
Use systematic nomenclature to write names and formulae for compounds containing species with variable oxidation states
Understand the terms ‘reduction’ and ‘oxidation’ in terms of electron transfer
Be able to identify a reaction as a REDOX reaction using changes in oxidation states
Write simple half-equations to show oxidation and reduction
Combine half-equations to form ionic (redox) equations
Understand what is meant by an oxidising agent and a reducing agent, identifying these in given reactions
Know that Group 7 are the Halogens (salt formers) and are diatomic
Know the electron configurations for F, Cl, Br,I
Know the trend in colour, state and electronegativity down Group 7, relating state to molecular and electronegativity to atomic structure
Explain the trend in reactivity down Group 7, linked to shielding, atomic radius and nuclear charge
Know what is observed in the displacement reactions between a
Halogen and a halide (including when hexane is present)
Explain the above displacement reactions
Write symbol, half and ionic (redox) equations for the above reactions
Identify the oxidising and reducing agents in the above reactions
Understand what the term ‘disproportionation’ means and use oxidation states to illustrate this
Write an equation for the reaction of chlorine with sodium hydroxide to make bleach
Write an equation for the reaction of chlorine with water to make chloric (I) acid (CARE: reversible)
Write an equation for the addition of chloric (I) acid to water to produce chlorate (I) ions (CARE: reversible)
Know that chlorate (I) ions kill bacteria so are used to treat water
Give some ‘for’ and ‘against’ arguments for adding chlorine to water
Give some ‘for’ and ‘against’ arguments for adding fluorine to water
Explain how nitric acid, aqueous silver nitrate and ammonia are used to identify halide ions
Write equations with state symbols for the above reactions
State and explain the trend in reducing power of the halide ions
-
Write equations and explain the observations for the reaction of
NaF and NaCl with H
2
SO
4
Write equations and explain the observations for the reaction of
NaBr with H
2
SO
4
Write equations and explain the observations for the reaction of
NaI with H
2
SO
4
Use oxidation numbers to discuss redox processes for the reactions of
NaX with H
2
SO
4