EXPERIMENT 12:
advertisement
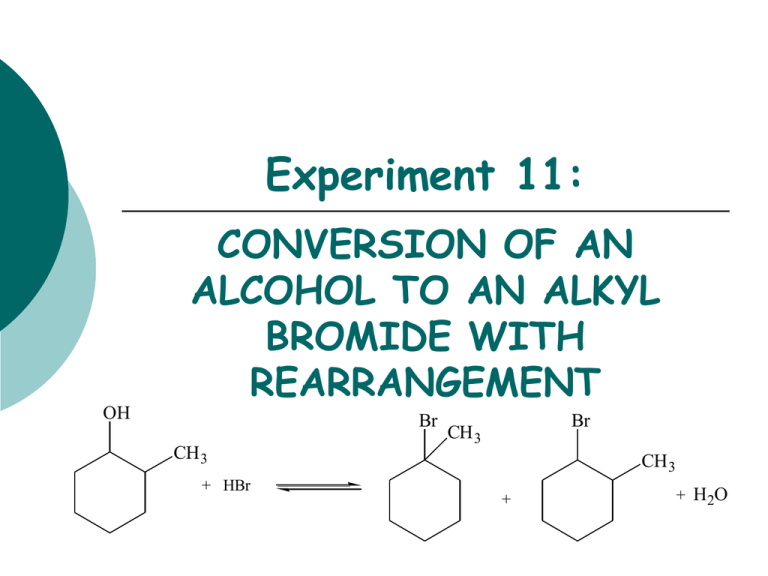
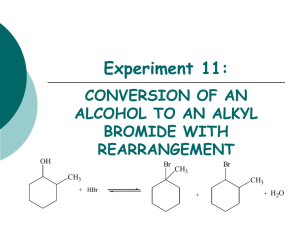
Experiment 11: OH CONVERSION OF AN ALCOHOL TO AN ALKYL BROMIDE WITH REARRANGEMENT Br CH 3 + HBr Br CH 3 CH 3 + + H 2O Objectives To synthesize isomeric alkyl halides from an alcohol using an SN1 dehydration reaction. To determine product substitution based on reaction rate with AgNO3. To characterize reactants and products using IR spectroscopy. CHEMICAL EQUATION OH Br CH 3 + HBr 2-methylcyclohexanol Br CH 3 CH 3 + 1-bromo-1-methylcyclohexanol (3o alkyl halide) + H2O 1-bromo-2-methylcyclohexanol (2o alkyl halide) SN1 MECHANISM 1. hydroxyl oxygen attacks and removes a proton from hydrobromic acid. H 2. Forms new O-H bond, oxygen bears a positive charge (oxonium ion). OH CH3 H H 3. Water is eliminated-forms 2o carbocation. H O Br H H H CH3 CH3 H H + H2O + Br secondary carbocation H H H H CH3 H H H H a a Br- CH3 Hydride Shift 4. Products may form from the 2o carbocation, but it is more likely that the 2o C+ will rearrange to a 3o C+. b b Br- Br 5. Reaction of the carbocation with the bromide ion yields the alkyl halide. Br CH3 CH3 5. Reaction of the carbocation with the bromide ion yields the alkyl halide. OVERVIEW Heat alcohol and acid under reflux to synthesize product(s). Purify product by extraction to remove unreacted starting materials. Obtain final product mass and calculate % yield. Analyze product with AgNO3 test to determine which product forms. EXPERIMENTAL PROCEDURE (Synthesis) Leave open • Add 2-methylcyclohexanol, 48% HBr and 3 boiling chips to flask. at top! water out • Clamp flask to ring stand. • Attach clear hoses to condenser and place condenser on top of flask. water in heating mantle to voltage regulator iron ring • Begin water flow through condenser and apply heat. • Reflux the solution for 30 minutes. • Cool flask in beaker of tap water. EXPERIMENTAL PROCEDURE (Purification) • Transfer solution to separatory funnel. • Add concentrated sulfuric acid. • Stir solution gently with glass rod. Do NOT agitate. • Draw off bottom acidic layer into an Erlenmeyer flask. • Transfer the top layer to a preweighed beaker using a glass Pasteur pipet. • Reweigh beaker to obtain final mass. • Perform silver nitrate test. Table 11.1 Must determine which reactant is your limiting reagent first, 48% HBr or 2-methylcyclohexanol. Calculate the amount of product that can be formed based on the limiting reagent. Theoretical Yield (g) This mass will be obtained by weighing an empty small beaker, then reweighing after the product has been added. The difference in mass is the actual product yield. Actual Yield (g) % Yield Product Appearance Actual yield (g) Theoretical yield (g) X 100 Physical state and color of product. EXPERIMENTAL PROCEDURE (Silver Nitrate Test) When an alkyl halide is treated with a solution of alcoholic silver nitrate, a precipitate of silver halide forms. The rate limiting step is the formation of the carbocation. Br + AgNO3 ethanol + C + NO 3- + AgBr(s) Rate of reaction: 3o > 2o > 1o Alkyl Bromides Table 11.2 Test Solution 1-bromobutane 2-bromobutane 2-bromo-2-methylpropane Product sample Reaction Rate (min/sec) You only need 3 drops of each of these! Get ONLY what you need! IR SPECTROSCOPY OH CH 3 Br CH 3 Table 11.3 Functional Group Base Values (cm-1) OH stretch 3200-3600 C-O stretch 1000-1200 sp3 CH stretch 2850-3000 C-Br stretch 500-700 2-methylcyclohexanol Alkyl halide product Frequency (cm-1) Frequency (cm-1) Infrared Spectroscopy (IR) (How to answer the questions…) How are you able to DIFFERENTIATE between reactant and product? Discuss the appearance of certain types of absorptions, or the disappearance of others, which indicate that functional groups have changed. Always answer like this: (fill in the blanks) In the IR spectrum of the product, the appearance of the _____ (type of bond) absorption at _____ (actual frequency) indicates the conversion of the reactant to the product. The typical frequency for this type of absorption is _____ (base value frequency). SAFETY CONCERNS Sulfuric acid is very corrosive and must be used with EXTREME caution! Silver nitrate will stain the skin! Use gloves at all times! Alkyl bromides are lachrymators! Keep covered at all times to avoid inhalation! WASTE MANAGEMENT Place the acidic solution from extraction in bottle labeled “AQUEOUS ACIDIC WASTE”. Place alkyl bromide product in the bottle labeled “ALKYL BROMIDE WASTE”. Pour the contents of the test tubes used in the silver nitrate test in the container labeled “SILVER NITRATE WASTE”. LABORATORY NOTEBOOK (Pre-lab) • OBJECTIVE (Must clearly state…) •What compounds you will make and how • How you will purify the compound • How you will determine the purity of your compound • CHEMICAL EQUATION •Include the general chemical equation from top of page 93. • TABLE OF PHYSICAL DATA (Complete the following table using MSDS sheets from a site on WWW Links ONLY. Wikipedia is unacceptable) • Compound 2-methylcyclohexanol 48% Hydrobromic acid Sulfuric acid 1-bromobutane 2-bromobutane 2-bromo-2-methylpropane Silver nitrate 1-bromo-1-methylcyclohexane 1-bromo-2-methylcyclohexane MW (g/mol) bp (Co) d (g/mL) XXX XXX XXX XXX XXX XXX REFERENCE TO PROCEDURE (Must include…) •full title, including edition and authors •page numbers where actual procedure can be found HAZARDS LABORATORY NOTEBOOK (In-lab) • DATA/CALCULATIONS • • • • • • • Initial weight of 2-methylcyclohexanol used Initial 50 mL beaker weight Final 50 mL beaker weight Final product weight Physical state and color of product Theoretical yield calculation (not just the number!) Percent yield calculation (not just the number!) • EXPERIMENTAL PROCEDURE • • In paragraph form, describe the procedure that you actually followed during the lab. Paragraph must be written in PAST TENSE, PASSIVE VOICE. • • Include ACTUAL volumes or weights of chemicals used during the experiment. Include any mistakes, accidents, or observations if necessary.