File - Ms Hotchin SCSC
advertisement

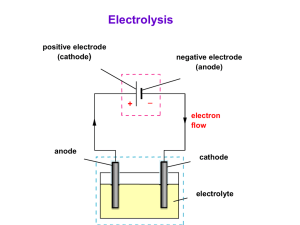
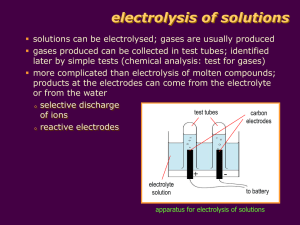
1. Introduction to electrolysis - electrolytes and non-electrolytes Electrolysis is the process of electrically inducing chemical changes in a conducting melton or solution e.g. splitting an ionic compound into the metal and non-metal. SUMMARY OF COMMON ELECTRICAL CONDUCTORS and what makes up the circuit? o These materials carry an electric current via freely moving electrically charged particles, when a potential difference (voltage!) is applied across them, and they include: o All metals (molten or solid) and the non-metal carbon (graphite). This conduction involves the movement of free or delocalised electrons (e charged particles) and does not involve any chemical change. o Any molten or dissolved material in which the liquid contains free moving ions is called the electrolyte and can conduct an electrical current. (see nonelectrolyte) Ions are charged particles e.g. Na+ sodium ion, or Cl- chloride ion, and their movement or flow constitutes an electric current, in other words the electrolyte consists of a stream of moving charged particles. What does the complete electrical circuit for electrolysis consist of? There are two ion currents in the electrolyte flowing in opposite directions positive cations e.g. sodium Na+ are attracted to the negative cathode electrode, and negative anions e.g. chloride Cl- are attracted to the positive anode electrode, The chemical changes occur at the electrodes which connect the electrolyte liquid containing ions with the external d.c. electrical supply. o If the current is switched off, the electrolysis process stops. Non-electrolytes are liquids or solutions that do not contain ions, do not conduct electricity readily and cannot undergo the process of electrolysis e.g. ethanol (alcohol), sugar solution etc. and are usually covalent molecules. Liquids that conduct must contain freely moving ions to carry the current and complete the circuit. o You can't do electrolysis with an ionic solid!, the ions are too tightly held by chemical bonds and can't flow from their ordered situation! o When an ionically bonded substances are melted or dissolved in water the ions are free to move about. However some covalent substances dissolve in water and form ions. e.g. hydrogen chloride HCl, dissolves in water to form 'ionic' hydrochloric acid H+Cl-(aq) The solution or melt of ions is called the electrolyte which forms part of the circuit. The circuit is completed by e.g. the external copper wiring and the (usually) inert electrodes like graphite (form of carbon) or platinum AND electrolysis can only happen when the current is switched on and the circuit complete. ELECTROLYSIS SPLITS a COMPOUND: o When substances which are made of ions are dissolved in water, or melted material, they can be broken down (decomposed) into simpler substances by passing an electric current through them. o This process is called electrolysis. o Since it requires an 'input' of energy, it is an endothermic process. During electrolysis in the electrolyte (solution or melt of free moving ions) ... o positive metal or hydrogen ions move to the negative electrode (cations attracted to cathode), e.g. in the diagram, sodium ions Na+ , move to the -ve electrode, o and negatively charged ions move to the positive electrode (anions attracted to anode), e.g. in the diagram, chloride ions Cl-, move to the +ve electrode. The diagram shows the industrial electrolysis process (in a Down's Process Cell) to extract sodium metal from sodium chloride (common salt). During electrolysis, gases may be given off, or metals dissolve or are deposited at the electrodes. o Metals and hydrogen are formed at the negative electrode from positive ions by electron gain (reduction), e.g. in molten sodium chloride sodium ions change to silvery grey liquid sodium, Na+ + e- ==> Na o and non-metals e.g. oxygen, chlorine, bromine etc. are formed from negative ions changing on the positive electrode by electron loss (oxidation), e.g. in molten sodium chloride chloride ions change to green chlorine gas, 2Cl- - 2e- ==> Cl2 o The electrons released by the oxidation at the positive anode, flow round through the anode and wire to the positive cathode and so bring about the reduction i.e. of the sodium ion. In a chemical reaction, if an oxidation occurs, a reduction must also occur too (and vice versa) so these reactions 'overall' are called redox changes. o You need to be able to complete and balance electrode equations or recognise them and derive an overall equation for the electrolysis. 2b. Summary of ELECTRODE REACTIONS - half-cell electrode equations Eq. no. (-) negative cathode electrode where reduction of the attracted positive cations is by electron gain to form metal atoms or hydrogen [from Mn+ or H+, n = numerical positive charge]. The electrons come from the positive anode (see below). (+) positive anode electrode where the oxidation of the atom or anion is by electron loss. Nonmetallic negative anions are attracted and may be oxidised to the free element. Metal atoms of a metal electrode can also be oxidised to form positive metal ions which pass into the liquid electrolyte. The released electrons move round in the external part of the circuit to produce the negative charge on the cathode electrode. 1 (-) Na + (l) + e- ==> Na(l) (sodium metal) 2 (+) 2Cl-(l/aq) - 2e- ==> Cl2(g) 3 The electrode equations are shown on the left with examples of industrial processes where this electrode reaction happens below on the right. Unless otherwise stated, the electrodes are inert i.e. they do not chemically change e.g. platinum or carbon-graphite. PLEASE NOTE - all electrode equations are a summarysimplification of what happens on an electrode surface in electrolysis. There may be e.g. two equations which are totally equivalent to each other to describe WHAT IS ACTUALLY FORMED e.g. the formation of hydrogen or oxygen and in some cases other products may be formed too. sodium ion reduced to sodium metal atoms: typical of electrolysis of molten chloride salts to make chlorine and the metal chloride ion oxidised to chlorine gas molecules: electrolysis of molten chloride salts(l) or their concentrated aqueous solution(aq) or conc. hydrochloric acid(aq) to make chlorine (-) 2H+(aq) + 2e- ==> H2(g) (hydrogen gas) or 2H3O+(aq) + 2e- ==> H2(g) + 2H2O(l) or 2H2O(l) + 2e- ==> H2(g) + 2OH(aq) hydrogen ion or water reduced to hydrogen gas molecules: electrolysis of many salt or acid solutions to make hydrogen All three equations amount to the same overall change i.e. the formation of hydrogen gas molecules and as far as I know any is acceptable in an exam? 4 copper(II) ion reduced to copper atoms: deposition of copper in its (-) Cu (aq) + 2e ==> Cu(s) (copper electrolytic purification or deposit) electroplating using copper(II) sulphate solution, electrode can 2+ - be copper or other metal to be plated 5 6 copper atoms oxidised to copper(II) ions: dissolving of (+) Cu(s) - 2e- ==> Cu2+(aq) (copper copper in its electrolytic dissolves) purification or electroplating (must have positive copper anode) (-) Al3+(l) + 3e- ==> Al(l) (aluminium) 7 (+) 2O 8 2- (l) - - 4e ==> O2(g) (oxygen gas) (+) 4OH-(aq) - 4e- ==> 2H2O(l) + O2(g) (oxygen gas) or (+) 2H2O(l) - 4e- ==> 4H+(l) + O2(g) (oxygen gas) Both equations amount to the same overall change i.e. the formation of hydrogen gas molecules and as far as I know either is acceptable in an exam? 9 (-) Pb2+(l) + 2e- ==> Pb(l) (lead deposit) 10 (+) 2Br-(l/aq) - 2e- ==> Br2(g/l) (bromine) hydroxide ions or water molecules are oxidised to oxygen gas molecules: electrolysis of many salt solutions such as sulphates, sulphuric acid etc. gives oxygen (chlorides ==> chlorine in concentrated solution, but can also give oxygen in diluted solution) lead(II) ions reduced to lead atoms: electrolysis of molten lead(II) bromide(l) bromide ions oxidised to gas/liquid bromine molecules: electrolysis of molten bromide salts(l) or their concentrated aqueous solution(aq) or conc. hydrobromic acid(aq) to make bromine + 2e ==> Zn(s) (zinc deposit) (-) Ag+(aq) + e- ==> Ag(s) (silver deposit) silver ions reduced to silver atoms: silver electroplating from silver salt solution(aq), electrode can be other metal (-) Zn 13 oxide ion oxidised to oxygen gas molecules: electrolysis of molten oxides e.g. anode reaction in the extraction of aluminium from molten bauxite. zinc ions reduced to zinc atoms: galvanising steel (the electrode) by electroplating from aqueous zinc sulphate solution, (or from molten zinc chloride?) 11 12 aluminium ions reduced to aluminium atoms: extraction of aluminium in the electrolysis of its molten oxide ore(l) 2+ (aq) - calcium ions reduced to (-) Ca2+(l) + 2e- ==> Ca(s) (calcium calcium atoms e.g. in molten metal) calcium chloride or bromide etc.