Restoration of Maritime Artifacts - slider-chemistry-12
advertisement

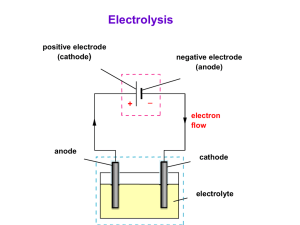
Restoration of Maritime Artifacts YEAR 12 CHEMISTRY SHIPWRECKS R. SLIDER Chloride and Sulfate Saturation Artifacts are often saturated with Cl- and SO42- ions as these are commonly found in sea water. Crystallisation: once an artifact has been brought to the surface, it can dry out allowing salts of chloride and sulfate to crystallise. This can destroy the cellular structure of wooden items as they dry. Soaking: to prevent the damage caused by these salts, wooden items need to soak for long periods in fresh water solutions to dissolve minerals. Electrophoresis Removal of charged particles by charged electrodes that attract the charged particles out of organic materials such as wood or pottery. - + ions - ions + + + + Concretions Artifacts can also be coated in deposits of sand and compounds such as CaCO3. These deposits often form hard, solid coverings known as concretions or encrustations. Concretions are commonly removed by soaking in 1M HCl or a weaker acid such as acetic acid. They may also be chipped away. The photographs show the concretion of a side plate from a firearm recovered from the ship Queen Anne’s Revenge. X-rays are used to identify the object and prevent damage when removing deposits. Source: http://www.qaronline.org/conservation/LabReportApril09.htm Restoration of Iron Artefacts Desalination: Chloride and sulfate compounds must again be prevented from crystallising, so the object is kept moist until they are removed by soaking. pH To prevent further corrosion due to potentially acidic conditions in the ocean iron artefacts are commonly soaked in slightly alkaline solutions of dithionate (S2O42-), which is reducing agent that prevents further corrosion. Dilute NaOH is also sometimes used which converts FeCl2 to Fe(OH)2 and releases chloride ions from the surface and forms a protective oxide layer. Electrolysis: iron or steel object is placed in dilute NaOH or Na2CO3 . The artefact is the cathode (-) where water is reduced: 2H2O + 2e- H2 + 2OHThe hydrogen gas helps loosen the calcareous deposits. The anode (+) is a steel mesh that attracts the negative chloride ions. Restoration of other metals Silver: Black Ag2S is a common compound found on silver artefacts. Electrolysis can be used to convert this compound back to silver metal. Again the artefact is the cathode (-) and the anode is stainless steel. Ag2S(s) + 2e- 2Ag(s) + S2-(aq) Copper, bronze, brass and lead can also be treated by electrolysis. CuS(s) + 2e- Cu(s) + S2-(aq) Leather Restoration 1. 2. 3. 4. 5. Water and physical means such as soft brushes and ultrasonic baths can be used to clean the object of dirt. A mild detergent may also be used. Any calcareous deposits can be removed with dilute ammonium citrate. Waterlogged leather is then treated with a polyethylene glycol (PEG) solution which softens the leather. Boric acid may be added to the above solution as a fungicide. Freeze-drying is then accomplished over a few weeks. This leather shoe was found off Newfoundland from a whaling ship that sank in 1565 Source: http://historytothepeople.ca/2010/06/doors-open-ottawa2010-parks-canada-conservation-laboratories/