comments on LO
advertisement

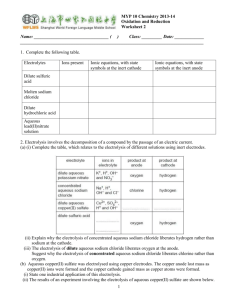
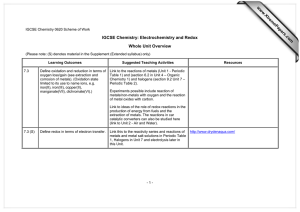
Assignment 3: Name: Langauon, Michele Mabel Esquiros School: Ramon Magsaysay (Cubao) High School Part 1: LO Title of LO Short description Ion Charges Ion charges are used to write the correct chemical formula of the compound. The applet is about the purification of copper. It is the electrolysis of copper(II) sulphate solution using copper electrodes to purify copper. It’s about the formation of diatomic molecules by covalent bonding showing how atoms are shared in covalent bonding to attain stability. It shows which of the two gases, NH3 and HCl diffuses faster relative to their atomic mass. After the two gases met, white NH4Cl is formed. It is an animation about the properties of the 3 states of matter, solid, liquid and gas on how the molecules in each state behave. Different indicators change its colour at different pH of the solutions. A glowing splint is placed in a test tube to test for the presence of oxygen gas. The glowing splint rekindles in the presence of oxygen gas. It describes and shows Electrolysis Covalent Bonds Rate of diffusion States of Matter Indicators Test for oxygen gas Fuel Cell Useful or not useful useful If useful, describe an activity how you can use it Writing chemical formula of ionic compounds. useful Electrolysis of solutions. (Different solutions will be used) useful How molecules are formed useful It can be used in the discussion of the diffusion of gases and molecular mass of gases. useful Comparison of the three states. The properties of matter might be presented in a table form to compare the 3 states of matter. useful Acid-Base Titration useful It can be used to test for the gas collected in the electrolysis of water. (The activity is electrolysis of water and after collecting the gas, use the oxygen test for the presence of oxygen gas) Building a galvanic cell that acts useful Dry ice Distillation how a galvanic and fuel cells work. It is the sublimation of dry ice, the phase change from solid to gas without passing the liquid phase. It is a method of purification of mixture to separate a volatile liquid from a non volatile solute. The solution is boiled to vaporize the volatile component and the vapour is cooled by cold water in the condenser to condense it back to a liquid (distillate) which is collected in the flask. useful useful as a battery and produces electricity It can be used as example of the phase changes in matter. The activity is all about the phase changes in matter. Distillation of salt solution