Oxidation-Reduction Chemistry Worksheet for MYP 10
advertisement
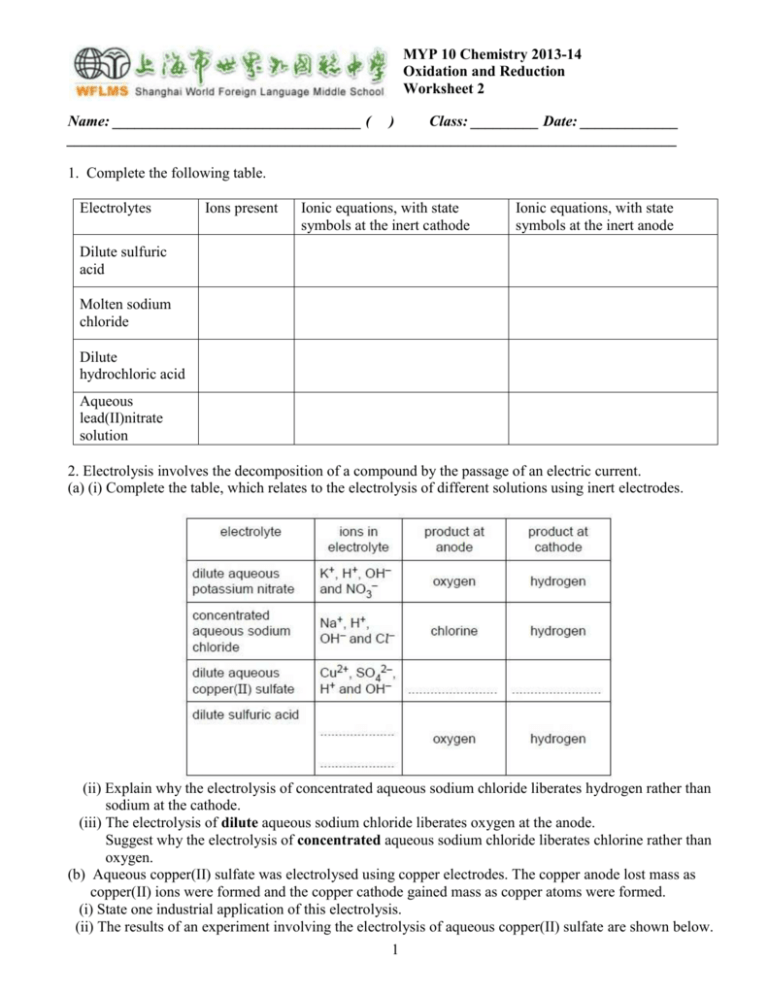
MYP 10 Chemistry 2013-14 Oxidation and Reduction Worksheet 2 Name: _________________________________ ( ) Class: _________ Date: _____________ _________________________________________________________________________________ 1. Complete the following table. Electrolytes Ions present Ionic equations, with state symbols at the inert cathode Ionic equations, with state symbols at the inert anode Dilute sulfuric acid Molten sodium chloride Dilute hydrochloric acid Aqueous lead(II)nitrate solution 2. Electrolysis involves the decomposition of a compound by the passage of an electric current. (a) (i) Complete the table, which relates to the electrolysis of different solutions using inert electrodes. (ii) Explain why the electrolysis of concentrated aqueous sodium chloride liberates hydrogen rather than sodium at the cathode. (iii) The electrolysis of dilute aqueous sodium chloride liberates oxygen at the anode. Suggest why the electrolysis of concentrated aqueous sodium chloride liberates chlorine rather than oxygen. (b) Aqueous copper(II) sulfate was electrolysed using copper electrodes. The copper anode lost mass as copper(II) ions were formed and the copper cathode gained mass as copper atoms were formed. (i) State one industrial application of this electrolysis. (ii) The results of an experiment involving the electrolysis of aqueous copper(II) sulfate are shown below. 1 Use the information in the table to describe how each of the variables affects the mass of copper formed at the cathode. temperature current time 3. A solution of iodide ions reacts with a solution of iron(III)chloride to produce iodine and a solution of iron(II)chloride. (a) Write a half equation to show the conversion of iron(III) ions to iron(II) ions. (b) Write a half equation to show the conversion of iodide ions to iodine. (c) Hence write an overall ionic equation for the above reaction. (d) In terms of electron gain or loss, explain which ion has been reduced. 4(a) Use these equations, which refer to aqueous solutions, to answer the questions that follow: Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) Cu(s) + 2Au+(aq) Cu2+(aq) + 2Au(s) Mg(s) + Fe2+(aq) Mg2+(aq) + Fe(s) (Au represents gold, which is below silver in the reactivity series) (i) List the metals above in order of decreasing reactivity. (ii) Define oxidation, in electronic terms, using one example from above. (iii) Define reduction, in terms of oxidation number, using one example from above. (iv) State and explain which is the strongest reducing agent in the examples above. (v) State and explain which is the strongest oxidizing agent in the examples above. (vi) Deduce whether a gold coin will react with aqueous magnesium nitrate. (b) Sketch a diagram of a cell used to electrolyse a molten salt. Label the essential components. (c) Describe how electrode reactions occur in an electrolytic cell and state the products at each electrode when molten copper(II)iodide is electrolysed. 2 5(a) Electrolysis can be used to obtain fluorine from molten potassium fluoride. Write an equation for the reaction occurring at each electrode and describe the two different ways in which electricity is conducted when the cell is in operation. (b) In one experiment involving the electrolysis of molten potassium fluoride, 0.1 mol of fluorine was formed. Deduce, giving a reason, the amount of potassium formed at the same time. (c) Sodium will displace aluminium from its chloride on heating: 3Na + AlCl3 3NaCl + Al (i) Explain, by reference to electrons, why the reaction is referred to as redox reaction. (ii) Deduce the oxidation numbers of sodium and aluminium in the reactants and products. 6. A voltaic cell is set up with a silver reference electrode and a series of other metals immersed in an electrolyte. The cell voltages were recorded in the table below. Metal Aluminium Zinc Iron Copper Silver Cell voltage / V 2.47 1.55 1.19 0.46 0.00 (a) What is the relationship between the voltage of the cell and the position of the metal in the reactivity series? (b) Is the metal acting as the negative or positive electrode? Explain your answer. (c) Construct the half-cell equations for a voltaic cell in which the metal is zinc and the electrolyte is silver nitrate. 3