Electrolysis of Copper Sulfate Worksheet
advertisement

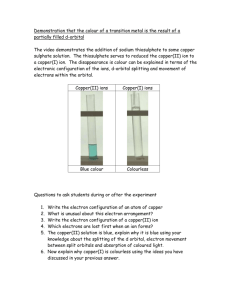
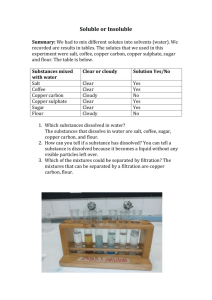
The Electrolysis of Copper Sulphate Label the electrolysis diagram with these labels: Pure Copper Cathode, Impure Copper Anode, Copper Ion (Cu2+), Copper (II) sulphate solution and then sketch how the 2 anodes will look after 4 hours. The impurities left behind at the impure copper anode from either: a) Sludge – impurities less reactive than copper stay as atoms and drop to the bottom of the tank eg. Gold b) Stay in Solution – impurities more reactive than copper stay as ions in solution but cannot get their electrons back as easily as copper ions. 1. Why would someone want to buy the sludge at the bottom of the electrolysis tank? 2. Describe the journey of the electrons and ions from the battery, through the solution and back to the battery.