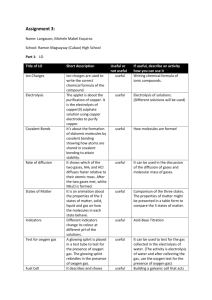
3. Electrolysis and Chemical Equations

3. Chemical Reactions
Water Electrolysis and Chemical Equations
Sue Boudreau 4.10.2012
Electrolysis means to split molecules with electricity. (Electro = electricity, -lysis = to split)
Use what you know about ions to predict what will happen in this set-up. Then learn how to balance
chemical equations.
1. Electrolysis of Water Experiment:
The set up:
Safety/Breaky Rules:
Wear safety glasses.
Keep power OFF until your set-up is complete and your fingers are NOT TOUCHING the water.
DO NOT short circuit your power supply. You will break it. Test it by touching the electrodes to a working bulb. a. Predict what you think will happen. b. Do it. Note exactly what really did happen. Include HOW MUCH of each gas was made.
2. Analysis:
a. Draw a molecule of water. Which will tend to attract electrons and is the –ive end of this polar molecule? Which is the +ive end? b. Explain what happened using vocabulary from electro chemistry (ions, electrolysis, electrode, +ive, ive)
When/where might it be useful to electrolyse water?
3. Figuring out chemical equation for the electrolysis of water: a. What were the REACTANTS? b. What were the PRODUCTS? c. Write the chemical equation for this reaction in WORDS: d. Write the chemical equation for this reaction using CHEMICAL SYMBOLS.
4. Use what you know about algebra to balance the equation:
Just like in an algebraic equation, the numbers of each kind of atom must be the same on each side of the equation. This is called “The Law of Conservation of Mass”. Molecules themselves can’t change the suit the equation: For example, oxygen goes around as O
2
, not as O
6
.
The NUMBER of each kind of molecule can change: If you have 6 oxygen ATOMS on the reactants side, you would write that as 3 O
2
which means three molecules of oxygen or 6 total atoms of oxygen.
Here’s a fictitious example: Q
2
+ T
4
-> 2QT
2 a. Use beads or model kits to model and balance this equation.
Use a different color of bead for each kind of atom. Use one prickle pad for the reactants and another for the products of the reaction. Do the number of Q atoms balance on each side of the equation?
____Do the number of T atoms balance? ____ b. Use the prickle pads and beads to model the equation for the electrolysis of water. Make sure the number of atoms of hydrogen and the number of oxygen atoms are the same for the reactants and the products.
Write the balanced equation for the electrolysis of water here:
5. Read Chapter 8.2. Do the review questions and/or the workbook to practice balancing equations.
Electrolysis of Water Teacher’s Notes:
VERY difficult to get the classic experiment to go. Even when I did, you only get Hydrogen coming off well and rust at the other electrode with very little gas. Add quite a bit of the salt catalyst – try sodium sulphate, sodium carbonate.
Have kids use either pencils sharpened at both ends or graphite rods. Use baking soda or sodium carbonate as the salt, but don’t tell ‘em. Keeps it simpler. Observe the bubbles. Which produces more?
Can easily see the difference between electrodes.
Show the YouTube of the classic experiment.
Try similar experiment they just did only this time, use a plastic container, add dish soap. Teacher demos exploding the bubbles. Maybe okay for kids. Try it first. Goggles. Use tongs for the lighted spill.
Great start to using the molecule model kits.
Nice lead in to hydrogen/oxygen explosions and gas testing.