LAB: Electrolysis of Water
advertisement

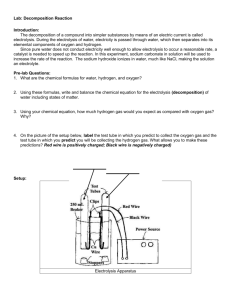
Name ________________________ Block ___ Date ___________ Chemical Changes Electrolysis of Water Introduction: The breakdown (also known as decomposition) of a compound into simpler substances by means of an electric current is called electrolysis. Water is a polar molecule meaning that one side of it is positively charged (hydrogen atoms) while the exact opposite side is negatively charged (oxygen atom). During the electrolysis of water, electricity from a battery is passed through water, which then separates the water molecule into its elemental components of oxygen and hydrogen. This happens because like the poles of a magnet, opposites attract. In other words, with electricity, positives attract negatives! The positive and negative ends of the battery literally pull apart the atoms of the molecule. Since pure water does not conduct electricity well enough to allow electrolysis to occur at a reasonable rate, something is needed to speed up the reaction. In this experiment, sodium carbonate dissolved in the water will be used to increase the rate of the reaction. The sodium carbonate ionizes in water, much like NaCl, making the solution an electrolyte. Pre-Lab: 1. The chemical or molecular formulas for water, hydrogen and oxygen are H2O, H2 and O2 respectively. Using these chemical formulas, write a chemical equation for the electrolysis of water. 2. Using the chemical equation you just wrote, form an hypothesis as to how much hydrogen gas will form as compared with oxygen gas? The sentence has been started for you – complete it. The hypothesis of the experiment is that there will be _______________ Procedure: 1. Setup as per teacher instructions 2. Glasses a must! Do not stick your hands in the solution. 3. In your journal, draw, title and label your own diagram of the apparatus. Record all observations. 4. Use rubber gloves to fill and invert test tubes in beaker 5. Upon completion of electrolysis follow teacher instructions for oxygen and hydrogen tests. Questions: 1. The black wire coming from the battery/transformer is the negative side of the “battery” while the red wire is the positive side. Compare the volumes of gases inside the test tubes to the negative and positive ends of the wires that are attached to the electrodes. Using your hypothesis written on the first page can you figure out which lead corresponds to which gas? Hint: Compare the numbers of molecules for hydrogen and oxygen that are predicted by the balanced chemical equation. Electrode Use a ruler to measure the length of the test tube that is filled with gas. Identity of the gas Need a ruler! Red (positive) Black (negative) 2. Which gas forms at the positive (red) wire and why? 3. Which gas forms at the negative (black) wire and why? 4. Write the balanced equation for the reaction of the larger volume of gas with oxygen in the air. Identify the reactants and the products. 5. Energy, in the form of electricity, was put into the reaction to break water apart into hydrogen and oxygen. What evidence showed that energy is released when water is formed from the hydrogen? 6. Did the results support or not support your hypothesis. Yes or no and explain. The result of this experiment _________________________________ 7. List the observations recorded in your journal that indicate that a chemical change is occurring. LAB: Electrolysis of Water Electrolysis Apparatus 2H2O 2H2 + O2 Water is a polar molecule meaning that one end (the hydrogen atoms) are positively charged (+) and the other end (oxygen side) is negatively (-) charged. The negative and positive charges on the ends of a battery can be used to attract the opposite charged atoms in a molecule such as water. In your journal 1. Compare the quantity of H being collected versus O. Does this make sense? Explain. 2. Describe how a scientist would test for oxygen and hydrogen. 3. Write the chemical equation for each test. 4. In an essay, explain the difference between a chemical change and a physical change. Give at least two examples of each. Use the back of this paper to outline a draft. Lab : Electrolysis Electrolysis Lab Extensions: □ 1. If time permits, repeat the collection of the gases. When one test tube is half filled, turn off the power supply, switch the electrical leads, and turn the power back on. □ 2. Collect as much of the mixture of gases as time will permit. Carefully test the mixture with a burning splint. □ 3. Try salt as an electrolyte. Be sure to check with your teacher before you do this as you might create a dangerous gas! Research and then explain what is happening. □ 4. Try using tap water instead of distilled water. What happens? Explain it!