Practical 2
advertisement

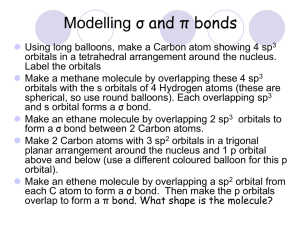
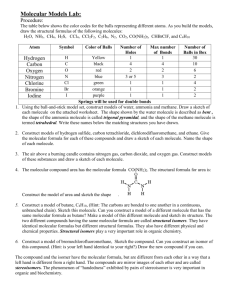
CM3109 Practical Use of ab-initio molecular electronic structure calculations to determine the vibrational frequency of a diatomic molecule. The purpose of this practical is: 1) to use the molecular electronic structure calculation, Gaussian 98, to calculate the Morse Potential of a diatomic molecule; 2) make an reasonable fit of a simple harmonic oscillator potential to the Morse potential 3) use the force constant of the SHO potential to estimate the vibrational frequency of the molecule and compare this and the estimated bond length with experimental values. 1) The Gaussian 98 calculation is a huge suite of programmes which will calculate the electronic structure of a molecule in terms of molecular orbitals. For the purpose of this practical the student needs to inform the calculation of the following input: a) The level of calculation to be used. There are two choices which are HartreeFock theory or Density Functional theory. The practical will use HF, although DFT is now the generally preferred method for many chemical calculations. b) The manner in which atomic orbitals will be represented. This is mathematically complicated but chemically quite simple. The molecular orbitals will be calculated in term of the atomic orbitals of the atoms. The calculation needs to know how many orbitals on each atom to include and how accurately these orbitals are to be described mathematically. These are specified in symbols such as STO-3G, 4-31G, 6-311G** (there are about 20 different commonly used variations on these) which convey a great deal of information but the details do not need to be understood to complete the practical. The practical will use 6-31G*. c) The composition and geometry of the molecule. This particularly simple for a diatomic molecule, e.g. for the molecule XY, the calculation needs to know the atoms X and Y and the bondlength R. Students will be instructed in how to input this information into the Gaussian 98 programme. The programme has a ‘front end’ which is supposed to make this process easy and this will be used. Experience has shown that most of the difficulty in this practical comes from managing this front end. Once the above data has been inputted, Gaussian 98 is instructed to perform the calculation and the output includes the energy of the molecule E(R) at the bond length, R, specified. The energy is calculated in atomic units (au). Students run the programme many times collecting E(R), R points in the range R = 0.2 … 2.4 Å, plotting the points, E(R) vs R (the use of EXCEL is recommended), to generate the Morse Potential. An approximate value of the minimum energy bond length of the molecule is determined from the plot and a number of additional points in its immediate neighbourhood are calculated. A (separate) plot of these points will have the appearance of a parabola and will provide an accurate estimate of the equilibrium bond length, Re. 2) This second plot of E(R) (au) vs R (Å) is approximated by a pure parabola (simple harmonic oscillator potential) V(R) = (k/2) (R – Re)2 . (It will be necessary to set the minimum of the E(R) (au) vs R (Å) plot to zero to do this.) The way in which this approximation is made is left to the student’s imagination, however using EXCEL and trial and error will yield a quick and possibly accurate result. There are more analytical approaches and for the enthusiast there is the Origin programme which will find the best possible fit – once the student has conquered its input! 3) The value of k determined in the above process is in units of (au Å-2). Convert the value to J m-2 ≡ N m-1 and use this to estimate the value of the vibrational frequency, , in cm-1. Compare the calculated values of Re and with the experimental values. (An opportunity to investigate www.nist.gov ?) The write up should include the Morse Potential plot properly and clearly labeled; the plot in the region of Re showing both the calculated points and the best fit SHO curve; a brief description of how the fit was made and the calculations and comparisons asked for in 3. 1 au of energy = 4.359 744 17(75)×10-18 J 1 Å = 10-10 m (1 / 2 ) k / s-1 / c k – force constant (N m-1) c – speed light (cm s-1) μ = reduced mass = m1m2 / (m1 + m2) (kg)