water
advertisement
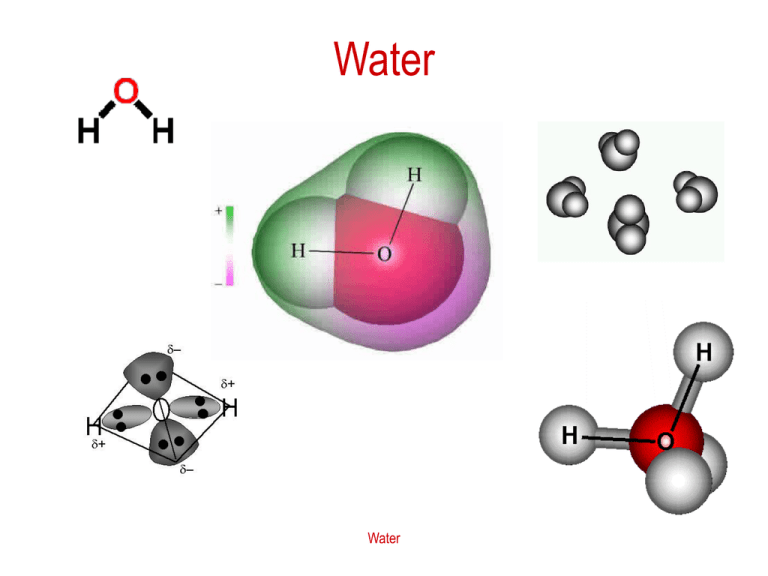
Water Water Vibration Spectrum of Water Water The Water Molecule The occupied molecular orbitals (as electron probability distributions of the isolated molecule) were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. They are set out with the lowest energy (most negative) molecular orbitals at the top. The calculated energies are -559 ev, -37 ev, -19 ev, 15 ev and -14 ev. It can be seen that the three highest energy orbitals are orthogonal around the oxygen atom, with two lowest energy orbitals (1s2 and mostly 2s2) approximately spherical (at the top). There are no obvious sp3 hybridization characteristics. The highest energy orbital (1b1) is predominantly pz2 in character and mainly contributes to the “lone pair” effects. These orbitals are appreciably changed in ice and water, with the 3a1 orbital being shown experimentally to contribute most to hydrogen bonding Water sbu.ac.uk/water/h2oorb.html Water Dimmers When two molecules come close to each other, hydrogen bonding holds them into a dimer, which may have one or two H-bonds, or converting from one to the other. Water Water Dimmer Water Ice at Atmospheric pressure At 1 atm, solid water is called ice I. There are two types: hexagonal Ih and cubic Ic. Hydrogen bonding features crystal structures of ice. Water Hexagonal Ice Ih Water Phase Diagram for H2O Water Amorphous solid H2O Water Phase diagrams of water Phase diagram for junior scientists and phase diagram including extreme conditions – very high pressure, at which other forms of ice are present. Water Absorption coefficient of Liquid Water Why is water blue? Water Dissolving HCl in Water The interaction of HCl molecule and water molecules is depicted here in a 2-dimensional, and let your imagination bring you the picture in 3D please. Water