Molecular Models Lab:
advertisement

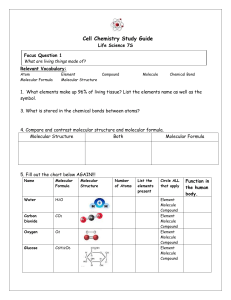
Molecular Models Lab: Procedure: The table below shows the color codes for the balls representing different atoms. As you build the models, draw the structural formulas of the following molecules: H2O, NH3, CH4, H2S, CCl4, CCl2F2, C2H6, N2, CO2, CO(NH2)2, CHBrClF, and C4H10 Atom Symbol Color of Balls Number of Max number Number of Holes of Bonds Balls in Box H Yellow 1 1 30 Hydrogen C black 4 4 10 Carbon O red 2 2 6 Oxygen N blue 3 or 5 3 2 Nitrogen Cl green 1 1 4 Chlorine Br orange 1 1 2 Bromine I purple 1 1 2 Iodine Springs will be used for double bonds 1. Using the ball-and-stick model set, construct models of water, ammonia and methane. Draw a sketch of each molecule on the attached worksheet. The shape shown by the water molecule is described as bent , the shape of the ammonia molecule is called trigonal pyramidal, and the shape of the methane molecule is termed tetrahedral. Write these names below the matching structures you have drawn. 2. Construct models of hydrogen sulfide, carbon tetrachloride, dichlorodifluoromethane, and ethane. Give the molecular formula for each of these compounds and draw a sketch of each molecule. Name the shape of each molecule. 3. The air above a burning candle contains nitrogen gas, carbon dioxide, and oxygen gas. Construct models of these substances and draw a sketch of each molecule. 4. The molecular compound urea has the molecular formula CO(NH2)2. The structural formula for urea is: Construct the model of urea and sketch the shape . 5. Construct a model of butane, C4H10, (Hint: The carbons are bonded to one another in a continuous, unbranched chain). Sketch this molecule. Can you construct a model of a different molecule that has the same molecular formula as butane? Make a model of this different molecule and sketch its structure. The two different compounds having the same molecular formula are called structural isomers. They have identical molecular formulas but different structural formulas. They also have different physical and chemical properties. Structural isomers play a very important role in organic chemistry. 6. Construct a model of bromochlorofluoromethane, Sketch the compound. Can you construct an isomer of this compound. (Hint: is your left hand identical to your right?) Draw the new compound if you can. The compound and the isomer have the molecular formula, but are different from each other in a way that a left hand is different from a right hand. The compounds are mirror images of each other and are called stereoisomers. The phenomenon of “handedness” exhibited by pairs of stereoisomer is very important in organic and biochemistry.