Hydrogen Spectrum: Electron Transitions & Wavelengths
advertisement
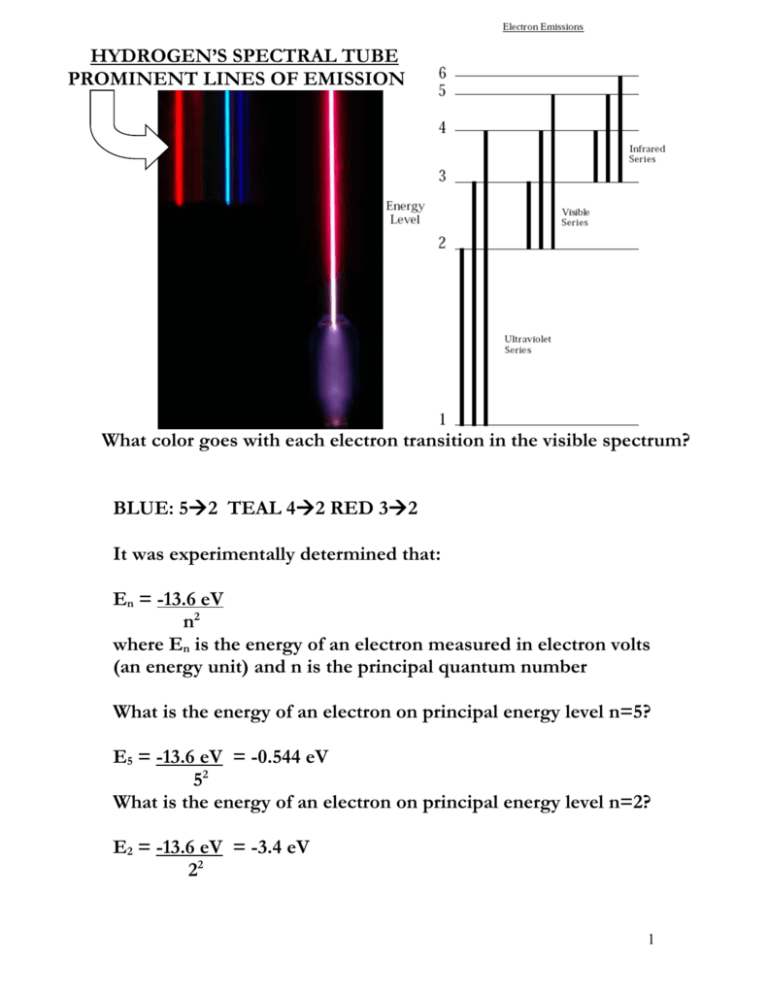
HYDROGEN’S SPECTRAL TUBE PROMINENT LINES OF EMISSION What color goes with each electron transition in the visible spectrum? BLUE: 52 TEAL 42 RED 32 It was experimentally determined that: En = -13.6 eV n2 where En is the energy of an electron measured in electron volts (an energy unit) and n is the principal quantum number What is the energy of an electron on principal energy level n=5? E5 = -13.6 eV = -0.544 eV 52 What is the energy of an electron on principal energy level n=2? E2 = -13.6 eV = -3.4 eV 22 1 If 1 eV=1.6 x 10-19 J (Joules which is another energy unit also involved in Planck’s constant), what are the values for E5 and E2 of hydrogen? E5 = -0.544 eV x 1.6 x 10-19 J = -8.704 x 10-20 J 1 eV E2 = -3.4 eV x 1.6 x 10-19 J = -5.44 x 10-19 J 1 eV What is the change in energy from E5 down to E2 ? (triangle is the Greek capital letter delta which means change) ΔE5 to 2 = Efinal – Einitial = E2 – E5 = -5.44 x 10-19 J – (-8.704 x 10-20 J) = -4.5696 x 10-19 J =-4.57 x 10-19 J Why is the value of this transition “negative energy?” Because the energy is being emitted as the electron falls from n=5 to n=2. 2 What is the wavelength of the photon of light emitted from E5 down to E2 ? IF: Equation 1 Equantum = h ν energy = Planck’s constant x frequency (6.626 x 10-34 J.s) AND: Equation 2 c = λ ν speed of light = wavelength x frequency (3.00 x 108 m/s) AND: Equation 3 ν = _c_ λ when equation 2 above is rearranged and solved for ν AND: Equation 4 Equantum = h c λ when ν in equation 1 is substituted by equation 3 THEN: Equation 5 λ = __h c__ Equantum when equation 4 is solved for λ λ5 to 2 = __h c__ E5 to 2 λ = (6.626 x 10-34 J.s)x (3.00 x 108 m/s) = -4.349671772 x 10-7 -4.57 x 10-19 J = -4.35 x 10-7 m Unit Cancelers 3 If 1.0 m =1.0 x 109 nm=1.0 x 1010 angstroms (Ǻ is another length value often used with atoms), what is the wavelength for the E5 and E2 transition of hydrogen in Ǻ and nm? λ = -4.35 x 10-7 m x 1.0 x 1010 Ǻ = -4.35 x 103 Ǻ = -4350 Ǻ 1.0 m λ = -4.35 x 10-7 m x 1.0 x 109 nm = -4.35 x 102 nm = -435 nm 1.0 m Unit Cancelers ACTUAL VALUE OF HYRDOGEN’S E5 to E2 TRANSITION λ5 to 2 = 434.17 nm Does this make sense? 4 HOMEWORK: 1) Show that the E4 to E2 transition should emit photons of about λ4 to 2 = 486.27 nm 2) Show that the E3 to E2 transition should emit photons of about λ3 to 2 = 656.47 nm 5











