Chapter 5 Review Sheet
advertisement

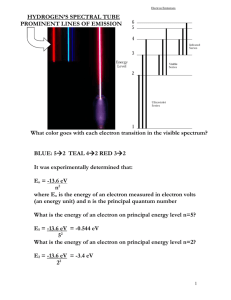
Chapter 5 Review Sheet ___E__ 1. hertz ___H__ 2. quantum of energy ___B__ 3. spectrum ___D__ 4. energy level ___G__ 5. atomic orbital ___I__ 6. amplitude ___J__ 7. wavelength ___C_ 8. atomic emission spectrum ___A__ 9. Aufbau principle ___F__ 10. frequency ___N__ 11. ground state __P___ 12. photon ___L__ 13. Heisenberg uncertainty principle ___K__ 14. Planck's constant ___M__ 15. de Broglie's equation Name ________________________ A. electrons enter orbitals of lowest energy first B. a range of colors seen when light passes through a prism C. lines of colored light obtained by passing the light emitted by an element through a prism D. the region around an atomic nucleus where electrons are likely to be moving E. the SI unit of frequency F. the number of wave cycles to pass a given point per unit of time G. a region in space where there is a high probability of finding an electron H. the amount of energy required to move an electron from one energy level to the next higher one I. the height of a wave from the origin to the crest J. the distance between crests of waves K. 6.6262 x 10-34 J s L. It is impossible to know both the velocity and the position of a particle at the same time. M. predicts that all matter exhibits wavelike motions N. the lowest energy level O. a process in which electrons are ejected by metals when light shines on them P. light quantum Answer the following questions or solve the following problems in the space provided. 16. For each group of sublevels, circle the one that fills last as electrons are added? a. 3p 2p 1s 2s 3s b. 4p 3p 2p 4s 4d 5s 17. What is the maximum number of electrons in the: a. 1s orbital 2 b. 3d orbital 10 c. 3d 4s 3p 3s 2s c. 4p orbital 6 16. Write the electron configuration for the potassium atom. [Ar] 4s1 17. Write the electron configuration for the antimony (Sb) atom. [Kr] 5s2 4d10 5p3 18. Write the electron configuration for the magnesium atom. [Ne] 3s2 19. Write the electron configuration for the molybdenum atom. [Kr] 5s2 4d4 20. What is the element with the electron configuration: 1s2 2s2 2p6 3s2 3p6 3d7 4s2 Co 21. What is the frequency of radiation that has a wavelength of 4.7 x 10-5 cm ? ν c 3.00e 8 6.38e14 Hz 7 λ 4.7e 21. Calculate the wavelength of a photon of blue light whose frequency is 6.3 x 1014 s-1. c 3.00e 8 4.76e 7 m 6.3e14 22. Find the wavelength of each of these transitions in the hydrogen atom. a) E = 2.04 x 10-18 J b) E = 1.63 x 10-18 J E 2.04e 18 3.08e15 34 h 6.626e c 3.00e 8 m 9.74e 8 m 3.08e15 s E 1.63e 18 2.46e15 34 h 6.626e 3.00e 8 m 1.23e 7 m 2.46e15 s 23. An inexpensive laser that is available to the public emits light that has a wavelength of 670 nm. What is frequency of the radiation? c c 3.0e18 4.48e15 670 24. Draw and label a wave with the following (amplitude, wavelength, crest) Wavelength Amplitude Frequency 25. When an electron in an excited state moves from n=6 to n=2, what wavelength of energy is emitted? 410=visible 26. What color of visible light will each line emit? __Blue____ line x, 434 nm = 4.34 x 10-7 m ___Red____ line y, 656 nm = 6.56 x 10-7 m __Violet____ line z, 410 nm = 4.10 x 10-7 m