The Bohr Model of the Hydrogen Atom
advertisement

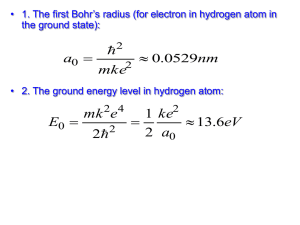
The Bohr Model of the Hydrogen Atom I. There is a state of the lowest energy – the ground state – which represents the normal condition of the electron as it orbits the nucleus. II. An atom can be excited when an electron is at greater distance from the nucleus than in the ground state. Energy of the excited state is higher than that of the ground one. III. The electron can exist only in certain sharply defined energy states, called orbitals. IV. There is a maximum energy that the electron can have and still be bound to the nucleus. V. An atom is not radiating energy in the form of light while the electron is on one of its orbitals. VI. Atom radiates energy only when the electron makes a transition from one orbital to a lower one. The radiation energy is the difference between the energies of the orbitals: Erad=Ek-Ej Examples: 1. Hydrogen atoms in states of high quantum number have been created in a lab. (a) Find the quantum number of the Bohr orbit in a hydrogen atom whose radius is 0.01 mm. (b) What is the energy of a hydrogen atom in this state? Solution: 5 m r 1 . 0 10 n 435 (a) n a 11 5.2910 0 E1 13.6eV 5 eV (b) En 2 7 . 19 10 n (435)2 2. Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H line. In the Balmer series the quantum number of the final state is nf=2. The longest wavelength corresponds to the smallest energy difference between energy levels. Hence the initial state must be ni=3. Then Solution: 1 R( 1 1 ) R( 1 1 ) 0.139R n2f ni2 22 32 1 6.56107 m; 0.139R This wavelength is near the red end of the visible spectrum. Examples: Find the de Broglie wavelength of (a) a 46-g golf ball with a velocity of 30 m/s, and (b) an electron with a velocity of 107 m/s. Solution: Both speeds are nonrelativistic: v«c. Hence: 34 J s h 6 . 63 10 4.81034 m (a) mv (0.046kg)(30m / s) The wavelength of the golf ball is so small compared with its dimensions that we would not expect to find any wave aspect in its behavior. 6.631034 J s 11m 7 . 3 10 (9.11031kg)(107 m / s) The dimensions of atoms are comparable with this figure—the radius of the hydrogen atom, for instance, is 5.31011 m. Thus, we need the wave properties of a moving electron to understand atomic structure and behavior. h (b) mv