Lecture 24: Quantum mechanics
advertisement

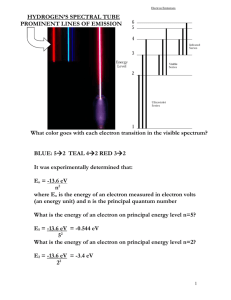
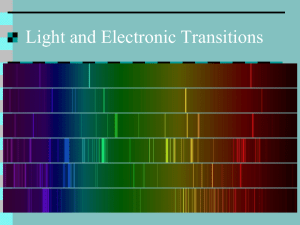
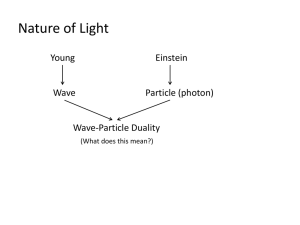
Lecture 24: Quantum mechanics Overview of Enzyme Kinetics The issue of vision Three experiments that changed physics o Black body radiation o Spectrum of Hydrogen o Photoelectric Effect Notions of quantum mechanics o o o o o o o Energy is quantized Light can be thought of as a particle Electron can be thought of as a Wave Wave-particle duality Location of a particle, how certain can we be? Heisenberg’s Uncertainty principle Probabilistic view of the microscopic world Vision revisited Black body radiation As we know, when we heat coal, initially it becomes red hot, and as it’s temperature increases further its color becomes almost white. The “spectrum” of light that is emitted by such body is shown below (Think of prism). More quantitatively, if we were to plot the intensity of emitted light as a function of wavelength it would look like the series of spectra shown to the left. Note it has a distinct maximum characteristic of its temperature. The position of the maximum shifts towards a lower wavelength (Blue shift) as the body temperature is increased. In the late nineteenth century, Raleigh showed that the energy density per unit wavelength should follow: I ( ) 8k BT 4 The corresponding simulated line is shown as dashed line in the above figure. Note the equation predicts intensity to increase continuously as the wavelength decreases. It does not predict a maximum at finite wavelength. This is known as ultraviolet catastrophe! Non-classical Explanation: Planck hypothesis. Planck assumed that the radiating substance was composed of electric dipoles that acted as simple harmonic oscillators. His suggestion was as follows: The energy of an oscillator must be discrete. E = n h where n is an integer, h is a constant of proportionality called Planck’s constant having value 6.6256 10-34 Js, and is the frequency of oscillation. The integer value of n cause the energy to be in multiples of h and results in discrete energy spectrum, as opposed to continuous energy spectrum of classical physics.Energy of the S.H oscillator following Planck’s hypothesis is said to be quantized, the allowed energy states are called quantum states, and the integer n is called the quantum number. His result yielded: Note the excellent fit with experimental results! The spectrum of hydrogen What is typically observed is a series of lines. If we were to calculate associated energies one finds: 1 1 E R 2 2 m n This is inconsistent with the classical picture. Niels Bohr explained this result by considering the angular momentum of electron-proton pair. He suggested, following Planck, that the angular momentum cannot be varied continuously, but it is quantized: nh i p r mvr 2 This is a very profound statement that can be experimental verified. Bohr’s Quantum Numbers and Spectroscopy To see how we can test above hypothesis, consider an electron and proton pair. Since the mass of the electron is much smaller than electron, let us assume that the electron revolves around proton with certain velocity v, tracing an orbit of radius r. However, to maintain an orbit we must balance centrifugal force, which can be due to Columbic interaction. Equating the centripetal force with coulombs law: mv 2 e 2 e2 2 F 2 v r mr r 2 but mvr nh 2 2 nh 1 nh 2 r v m 2 e 2 mr 2 4 2 2 2 me e e 2 E T V 1 mv 1 2 2 r r n2h2 Using this equation it’s possible to explain the entire absorption spectrum of Hydrogen! But it also asserts o Electron orbit radius is also quantized (r—n2) o Electron energy is also quantized consistent with Planck theory Photoelectric effect It was observed that the kinetic energy of the electrons ejected from clean metal surface by illuminating it with light follows somewhat remarkable behavior: In classical theory, the kinetic energy should be proportional to the intensity of light. Thus Einstein suggested following simple equation: EKE hv In this way he proposed that light can act as a particle; back to Newton again! Wave-particle duality If the light photon can act like particle then it would appear that particles such as electron should exhibit wave characteristic. These ideas were succinctly unified by de Broglie, who suggested that the electron wavelength be: h h p mv Question then arose how do we prove the wave nature of electrons. In a brilliant experiment, Stern and Gerlach proved this. They exploited Huygen’s principle that was used to prove light is wave. The primary effect of wave nature is the observation of diffraction or interference phenomena. Using single crystals of Nickel, and beam of electrons they observed the diffraction caused by single crystals just as in case of X-rays! A corollary of dual nature of light is that if it is particle then it must have mass. We can use Einstein relation to estimate a mass of photon: E mc 2 hv m hc h c These developments generated tremendous excitement in physics. Because this meant we have to discard classical mechanics, developed by Newton, to describe strange new properties at microscopic scale.