Name: Date: Period: ______ Flame Test Experiment: Atomic
advertisement

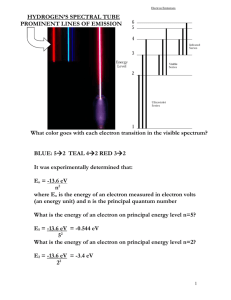
Name: _____________________________________________ Date: ______________________ Period: ________ Flame Test Experiment: Atomic Emissions Purpose: Upon completion of this experiment, students will be able to list the flame color of several different metals. They will be also be able to calculate the energy involved in the movement of electrons in the metals. Background / Introduction: When atoms or ions in the ground state are heated to high temperatures, some electrons may absorb enough energy to allow them to “jump” to higher energy levels. These excited state electrons are unstable and they will “fall” back to their normal positions of lower energy. As the electrons return to the ground state, the energy that was absorbed is emitted in the form of electromagnetic energy. Some of this energy may be in the form of visible light (colors). The color may be described in terms of its wavelength, and the following equation may be used to calculate the energy of the emitted photon: ∆𝐸 = ℎ𝑐 𝜆 ∆E is the difference in energy between the two energy levels in joules (J), h is Planck’s constant (6.626x10 -34 J*sec), c is the speed of light (3.0x108 m/sec), and λ is the wavelength of light in meters. The wavelengths are typically given in units of nanometers, nm, (1 meter = 1,000,000,000 nanometers). Each metal has its own specific color due to the amount of electrons and where they are spaced in the atom. Flame colors and the energy changes are used to help in identifying unknown substances, a qualitative analysis technique. The visible portion of the electromagnetic spectrum ranges from about 400 to 700 nm. The table below shows the approximate values for each observable color. Procedure: Record the compound name from the board. Each solution is set up at tables along with a burner. Move from one table to another making sure that you DO NOT CHANGE ANY OF THE WIRES FOR EACH SOLUTION!! Clean the wire for the solution by placing it in the flame until no color is seen. Dip the cleaned wire into the solution and place the tip of the wire into the flame, noting the color of the flame. Record this color and solution. Clean the wire and place it back down at each station. Based on the color of light emitted, record the corresponding wavelength listed in the provided table. Calculate the frequency and energy of light emitted using our equations from class. Repeat the steps for each of the supplied solution. Materials, Safety Information, etc. should be included as you go in this lab Color Violet Blue Green Yellow Orange Red Wavelength of Light λ (m) 4.20 x 10-7 4.75 x 10-7 5.40 x 10-7 5.80 x 10-7 6.05 x 10-7 6.35 x 10-7 Given Information / Equations: c = λν c = 3.0x108 m/s λ wavelength ν frequency ∆E = hν ∆E Energy h = “Planck’s Constant” Compound Color of Light Wavelength of Light, λ (m) Frequency of Light, ν (Hz) Energy, E (J) 1 2 3 4 Data Analysis: Electron Configuration 1. The metal in compound 1 is __________________________________________. Write the electron configuration for the metal ______________________________________________________________________________________ 2. The metal in compound 2 is __________________________________________. Write the electron configuration for the metal ______________________________________________________________________________________ 3. The metal in compound 3 is __________________________________________. Write the electron configuration for the metal ______________________________________________________________________________________ 4. The metal in compound 4 is __________________________________________. Write the electron configuration for the metal ______________________________________________________________________________________ Post Lab Question: 1. Reflecting on the atomic emission spectroscopy lab and this demonstration, what is occurring at the atomic level that causes visible light to be emitted when energy is applied? ______________________________________________________________________________________ ______________________________________________________________________________________ ______________________________________________________________________________________ ______________________________________________________________________________________ ______________________________________________________________________________________