doc
advertisement

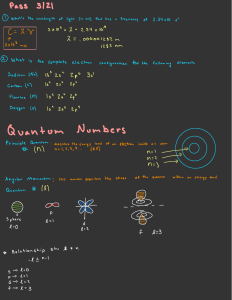
PHYS212-071 HW # 10 Solutions (Chapter 8) (Numbers refer to 2nd Edition of Textbook) 3. How many different sets of quantum numbers are possible for an electron for which (a) n = 1, (b) n = 2, (c) n = 3, (d) n = 4, and (e) n = 5? Check your results to show that they agree with the general rule that the number of different sets of quantum numbers is equal to 2n2. (a) n = 1, l = 0, ml = 0, ms = 1/2, +1/2, Total of 2 states, 212 = 2 (b) n = 2, l = 0, ml = 0, ms = 1/2, +1/2 l = 1, ml = 1, 0, + 1, ms = 1/2, +1/2 Total of 2 + 6 = 8 states, 222 = 8 (c) n = 3, l = 0, ml = 0, ms = -1/2, +1/2 l = 1, ml = 1, 0, + 1, ms = 1/2, +1/2 l = 2, ml = 2, 1, 0, + 1, + 2, ms = 1/2, +1/2 Total of 2 + 6 +10 = 18 states, 232 = 18 (d) n = 4, l = 0, ml = 0 l = 1, ml = 1, 0, + 1, ms = -1/2, + 1/2 l = 2, ml = 2, 1, 0, + 1, + 2, ms = 1/2, +1/2 l = 3, ml = 3, 2, 1, 0, + 1, + 2, + 3, ms = 1/2, +1/2 Total of 2 + 6 + 10 + 14 = 32 states, 242 = 32 (e) n = 5, l = 0, ms = 1/2, +1/2 l = 1, ml = 1, 0, + 1, ms = 1/2, +1/2 l = 2, ml = 2, 1, 0, + 1, + 2, ms = 1/2, +1/2 l = 3, ml = 3, 2, 1, 0, 1, + 2, + 3, ms = 1/2, +1/2 l = 4, ml = 4, 3, 2, 1, 0, +1, +2, +3, +4, ms = 1/2, +1/2 Total of 2 + 6 +10 + 14 +18 = 50 states, 252 = 50 10. Consider a single-electron atom in the n = 2 state. Find all possible values for j and mj for this state. n = 2, l =0, j = 1/2, mj = 1/2, +1/2 l = 1, j = 1/2, mj = 1/2, +1/2 j = 3/2, mj = 3/2, 1/2, +1/2, +3/2 11. Find all possible values of j and mj for a d electron. l = 2, j = 3/2, mj = 3/2, 1/2, +1/2, +3/2 j = 5/2, mj = 5/2, 3/2, 1/2, +1/2, +3/2, +5/2 12. Give the spectroscopic notation for the following states: (a) n = 7, l = 4, j = 9/2; (b) all the possible states of an electron with n = 6 and l = 5. (a) 7G9/2 (b) 6H9/2, 6H11/2 1