Exam 2 Review(sol)
advertisement

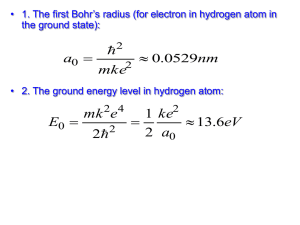
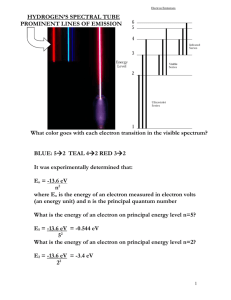
Review Exam #2 1. A monochromatic beam of light is absorbed by a collection of groundstate hydrogen atoms in such a way that six different wavelengths are observed when the hydrogen relaxes back to the ground state. (a) What is the wavelength of the incident beam? (b) What is the longest wavelength in the emission spectrum of these atoms? (c) To what portion of the electromagnetic spectrum does it belong? (d) To what series does it belong? (e) What is the shortest wavelength? (f) To what series does it belong? Solution: (a) The batch of excited atoms must make these six transitions to get back to state one: 2 1 , and also 3 2 and 3 1 , and also 4 3 and 4 2 and 4 1 . Thus, the incoming light must have just enough energy to produce the 1 4 transition. It must be the third line of the Lyman series in the absorption spectrum of hydrogen. The absorbing atom changes from energy Ei 13.6 eV 13.6 eV 13.6 eV E 0.850 eV f to 12 42 so the incoming photons have wavelength = c/f = hc/Ephoton 6.626 1034 J s 3.00 108 m s 1.00 eV hc . 19 E f Ei 0.850 eV 13.6 eV 1.60 10 J 9.74 108 m 97.4 nm (b) The longest of the six wavelengths corresponds to the lowest photon 13.6 eV 0.850 eV energy, emitted in the 43 transition. Here Ei 42 and Ef 13.6 eV 1.51 eV , 32 so hc 1240 eV nm 1.88 m Ef Ei 0.850 eV 1.51 eV . This infrared wavelength is part of the Paschen series, since the lower state has n = 3. (c) The shortest wavelength emitted is the same as the wavelength absorbed: 97.4 nm, ultraviolet, Lyman series. 2. (a) How much energy is required to cause an electron in hydrogen to move from the n = 1 state to the n = 2 state? (b) Suppose the electron gains this energy through collisions among hydrogen atoms at a high temperature. At what temperature would the average kinetic energy 3kBT/2, where kB is the Boltzmann constant, be great enough to excite the electron? Solution: (a) The energy difference between these two states is equal to the energy that is absorbed. Thus, E E2 E1 (b) 3 E kBT 2 13.6 eV 13.6 eV 4 1 10.2 eV 1.63 1018 J 2 1.63 1018 J 2E T 7.88 104 K 23 3kB 3 1.38 10 J K 3. Suppose the ionization energy of an atom is 4.10eV. In the spectrum of this same atom, we observe emission lines with wavelengths 310nm, 400 nm, and 1377.8 nm. Use this information to construct the energy level diagram with the fewest levels. Assume that the higher levels are closer together. Sketch this energy level diagram. Solution: E hc 1 240 eV nm 1 310 nm , 2 400 nm , 3 1 378 nm , E so E1 4.00 eV E2 3.10 eV E3 0.900 eV and the ionization energy 4.10 eV . The energy level diagram having the fewest levels and consistent with these energies is shown below 4. The positron is the antiparticle to the electron. It has the same mass and a positive electric charge of the same magnitude as that of the electron. Positronium is a hydrogen-like atom consisting of a positron and an electron revolving around each other. Using the Bohr model, find the allowed distances between the two particles and the allowed energies of the system. Solution: Let r represent the distance between the electron and the positron. The two r move in a circle of radius around their center of mass with opposite 2 velocities. The total angular momentum of the electron-positron system is quantized to according to Ln n 1,2,3, where For each particle, m vr m vr n 2 2 . kee2 F m a expands to n v We can eliminate m r to find r2 m v2 r2 . kee2 2m n2 2 r m 2r2 . So the separation distances are 2n2 2 2 r 2 a n 0 m kee2 1.06 10 10 m n2 . r 2 The orbital radii are 2 a0n , the same as for the electron in hydrogen. The energy can be calculated from 1 2 1 2 kee2 E K U mv mv 2 2 r Since kee2 mv , 2r 2 . kee2 kee2 kee2 kee2 6.80 eV E . 2r r 2r 4a0n2 n2