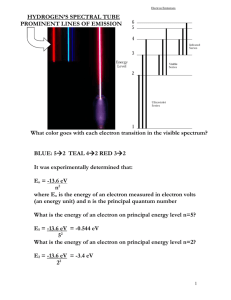
File
advertisement
THE COMPTON EFFECT Energy and momentum are conserved when a photon collides with an electron. KE=1/2mv2 X-ray E1 E2 E1 = E2 + KE(electron) EXAMPLE A) A Photon having an energy of 3.8eV collides with an electron at rest. The electron has a gain in kinetic energy of 1.2eV, what will be the energy of the emitted photon in eV and joules? E1 = E2 + KE(electron) 3.8eV = E2 + 1.2eV E2= 2.6eV E2 = 4.16x10-19J *(1 eV = 1.6 x 10-19J)* B) What is the frequency of the scattered light wave and what color is it? E2 =hf 4.16x10-19J= 6.63 x 10-34Js (f) f = 6.23 x 1014Hz color = blue c) What is the velocity of the electron? KE= 1/2mv2 1.2eV(1.6 x 10-19J/eV)= ½ (9.11x10-31kg)v2 1.92 x 10-19J = 4.56x10-31(v2) 4.2 x 1011 = v2 v= 6.49 x 105m/s DeBroglie Wavelength If light has particle properties then particles must have wave properties λ= h = h mv ρ λ- wavelength h- Planks constant m- mass v-velocity ρ- momentum EXAMPLE 1 • What is the DeBroglie wavelength of a 0.25kg ball thrown at 20m/s? λ= h mv = 6.63 x 10-34 Js (0.25kg)(20m/s) λ =1.33 x 10-34m *This is too small to observe.* EXAMPLE 2 What is the DeBroglie wavelength of an electron traveling at 1x106m/s? λ= h mv = 6.63 x 10-34Js (9.11x10-31kg)(1x106m/s) λ= 7.29 x 10-10m * Suitable for interference and diffraction and can be observed* EXAMPLE 3 • What is the momentum of a proton having a DeBroglie wavelength of 2.81x10-11m? λ=h/ρ 2.81x10-11m = 6.63 x10-34Js ρ ρ = 2.36x10-23kgm/s Heisenberg Uncertainty Principle • “To study the nature and motion of a moving electrons by bombarding them with photons would change the motion and position of the electron and lead to uncertainty” • You can’t accurately measure both position and momentum of a moving electron at any given time.