Phase Changes Worksheet: Melting, Boiling, Condensation
advertisement

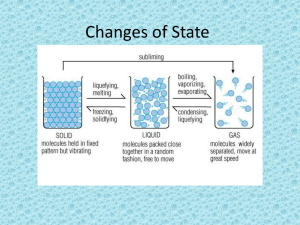
Phase Changes Name________________________________ Period_______ Date____________________ E VAPOR D CONDENSATION C BOILING B FREEZING A LIQUID MELTING SOLID 1. Does the temperature increase during melting? _____________ 2. Is energy required for both melting and boiling? _____________ 3. Can both liquid water and steam exist at 100°C? _____________ 4. What must be changed, temperature or heat energy during condensation? How? ____________ 5. How would you describe the change in arrangement of particles as heat energy and temperature increase from solid to liquid for water? _______________________________________________ 6. How would you describe the change in arrangement of particles as heat energy and temperature increase from liquid to vapor for water? ______________________________________________ 7. During melting & boiling temperature __________________________________ and heat energy _______________, during condensation and freezing temperature _________________________ and heat energy __________________. 8. After ice melts, what happens to the temperature of the water between its melting and boiling point? ______________________ 9. Which contains more heat, boiling water or steam (vapor)? _______________________________ 10. On the diagram as you move from point A to point E, what happens to the motion of the particles? ___________________________________ 11. At point D, what changes during boiling, temperature or heat energy? ______________________ 12. What happens to the density of water as it moves from point A to point C? __________________ 13. What happens to the density of water as it moves from point C to point E? __________________ 14. Are the particles at point A totally motionless? __________ 15. Name the letter where the particles are moving the fastest (have the most kinetic energy)_______ Two students performed the following experiment. They heated a beaker of ice and measured its temperature every one minute. After the ice melted, they continued measuring the temperature until it remained constant for a period of time. This is the graph of their results: °C 16. What is the starting temperature? ____________ 17. How long did this temperature remain constant? ____________ 18. What was the final temperature? ____________ 19. What is happening in the beaker at 0°C? ___________________ at 100°C? __________________ 20. At these temperatures, what was happening to the heat energy being added to the system? _______________________________________________________________________________ _______________________________________________________________________________ 21. How long did it take the students to reach 50°C ? ____________ 22. What was the temperature after 8 minutes? ____________ 23. How much did the temperature rise between 8 and 17 minutes? ____________