Matter Test Review Sheet
advertisement

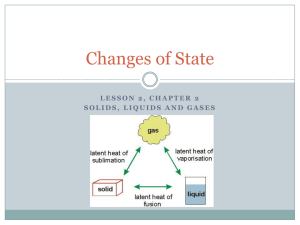
Physical Science Matter Review Name ________________________ Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Matter is defined as anything that a. can be seen and touched. b. has mass and takes up space. c. can be weighed. d. contains kinetic or potential energy. 2. A substance that cannot be broken down into simpler substances is a. a compound. c. an element. b. a mixture. d. an atom. 3. A material that can be represented by a chemical formula is a. an element. c. a homogeneous solution. b. a mixture. d. a pure substance. 4. Knowing the chemical properties of a substance will tell you how the substance a. looks. c. can be broken down into atoms. b. smells. d. reacts with other substances. 5. A liquid changes rapidly into a gas at the liquid’s a. boiling point. c. melting point. b. freezing point. d. condensation point. 6. A physical property of gold is its a. density. b. reactivity with powerful acids. c. non-flammability. d. None of the above 7. Grinding quartz crystals down to produce sand is an example of a a. change of state. c. chemical reaction. b. chemical change. d. physical change. 8. The process of a liquid becoming a solid is called a. condensation. c. evaporation. b. freezing. d. melting. 9. Which state of matter has a definite volume, but not shape? a. plasma c. liquid b. gas d. solid 10. Which state of matter will hold its shape without a container? a. solid c. gas b. liquid d. plasma 11. The kinetic theory states that the higher the temperature, the faster the a. particles that make up a substance move. b. bonds between atoms break down. c. molecules of gas rush together. d. lighter particles within a substance clump together. 12. The law of conservation of mass states that mass cannot be a. burned. c. created or destroyed. b. changed in form. d. heated or cooled. 13. Ice floats in water because it is a. more dense than water. b. less dense than water. c. colder than water. d. warmer than water. Completion / Short Answer Complete each sentence or statement. 14. Identify each of the following materials as an element, a compound/molecule, a homogenous mixture, or a heterogeneous mixture. ________________a. copper ________________b. salt water ________________c. carbon dioxide ________________d. apple ________________e. oxygen ________________f. gasoline 15. What physical properties could you use to tell the difference between each of the following kinds of matter? (One property per pair is sufficient) ________________a. coal and snow ________________b. vinegar and water ________________c. lead and copper ________________d. strawberry and vanilla ice cream ________________e. salt and sugar 16. Label the following statements as a physical change(PC), a chemical change(CC), a physical property(PP) or a chemical property(CP). ________________a. Donuts smell sweet. ________________b. Our big snow pile melted. ________________c. Hydrogen Peroxide bubbles inside a cut. ________________d. Alcohol has the ability to ignite. 17. Which physical property do all of the following have in common: glass, water, carbon dioxide, plastic wrap and air? 18. Aluminum, oxygen, and carbon are examples of ____________________. 19. A substance that is made of atoms of more than one type bonded together ionically is called a ____________________. 20. Every chemical compound is unique and different from the ____________________ it is made from. 21. The density of ____________________ is 1.0 g/cm3. 22. The ____________________ of a substance is defined as its mass divided by its volume. 23. A ____________________ property of a material can be observed without chemically changing the material. 24. A chemical change is a change in the ____________________ of a substance. 25. A change in color during a reaction is often a sign that a __________ __________ has occurred. 26. The kinetic theory states that the particles in matter are always in ____________________. 27. For any change of state to occur, ____________________ must be transferred. 28. What are the three types of pure substances and the two types of mixtures from your notes (not examples)? 29. Is crumpling a sheet of paper a physical or chemical change? Why? 30. Explain why changes of state are physical changes. 31. Which state of matter has particles able to slide past each other, yet stay packed together? 32. List four of the five evidences that suggest a chemical change is taking place. In the following problems list given, formula, setup, & solution & don’t forget units! 33. Calculate the density of a sample of gas with a mass of 30 g and volume of 7500 cm3. 34. Calculate the mass of a gas with a density of 0.0065 g/cm3 and volume of 260 cm3. 35. Calculate the volume of a liquid with a density of 1.7 g/ml and a mass of 144.5 g. 36. What is the boiling point of water (include units)? _____________________ 37. What is the melting temperature of ice (include units)? __________________ 38. What is the condensation point of steam (include units)? __________________ 39. What is the freezing temperature of water (include units)? __________________ Heating & Cooling Curve of Mercury Use the heating/cooling curve of mercury provided on the backside to answer the following questions ( don’t forget the scale units): 43. What is the freezing point of mercury? ________ 340 260 Temperature (C) 42. What is the condensation point of mercury? _____ 380 300 40. What is the boiling point of mercury? _________ 41. What is the melting point of mercury? _________ 420 220 180 160 120 Use the data table below to answer the following questions 80 The students in a class conducted an experiment to test how 40 long it would take for a substance to move through the different phases of matter. The students placed a test tube of 0 -40 a solid substance with a thermometer in it over a burner. They recorded the temperature of the substance every minute 0 until the substance disappeared from the test tube. The following is a data table from their experiment. 44. What is the independent variable? __________________________________________ 5 10 15 20 Time (min) Time (min) 0 Temp (K) 268 Time (min) 10 Temp (K) 293 Time (min) 20 Temp (K) 373 1 271 11 300 21 373 2 273 12 310 22 373 47. What is the control group in this experiment? __________________________________________ 3 273 13 320 23 373 4 273 14 333 24 373 48. What should be done to verify the results of this experiment? ________________________________ 5 273 15 345 25 373 6 274 16 357 26 373 49. From the data and your knowledge of the heating and cooling curves of substances describe what was happening around minutes 2-5? ___________________________________________ 7 275 17 369 27 8 280 18 371 28 9 286 19 372 29 45. What is the dependent or responding variable? __________________________________________ 46. What should be held constant in this experiment? __________________________________________ 50. What was happening from minute 20 through minute 26? ____________________________ 51. Why did they stop taking data after minute 26? ____________________________________ 52. Using the given data, infer what the condensation point temperature would be. ___________ 53. Using the data, infer what the freezing point temperature would be _____________________