naming compounds
advertisement

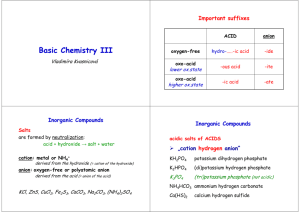
Naming Compounds Graphic Organizer and Practice Problems Organic Naming Compounds -if it’s a metal Check 1st Element -if it’s a H -if it’s a nonmetal Ionic Molecular Acid Split into ions Check charge for transition metals Cation- same as element -ide(anion), -ate(anion), hydro____ic acid ____ic acid Anion- -ide or polyatomic Put together ions so net charge is 0 -if you have C and H -ite(anion), ____ous acid # of H’s match the charge of the anion *Name elements from left to right using the periodic table *use prefixes to tell how many *end in -ide Practice Part 1 FeS NCl3 First element is a metal, so it is ionic First element is a nonmetal, so it is molecular Cation would be iron Anion would be sulfide Iron (II) sulfide Nitrogen trichloride Practice Part 1 (cont.) CoSO4 H3PO3 First element is a metal First element is H, so it is an acid Cobalt (II) Sulfate Normally would end in -ite, so should be an -ous acid Phosphorous acid Practice Part 2 potassium sulfide Hydrobromic acid First element is a metal, so it is ionic Its is a hydro __ic acid, so it should end with -ide Potassium ion is K+ Sulfide ion is S2 To balance charges it should be K2S Bromide has a -1 charge HBr Practice Part 2 (cont.) Tin (IV) chloride Dinitrogen pentoxide Starts with a metal, so it is ionic Uses prefixes so it is molecular (IV) means tin has a +4 charge Di means it has 2 nitrogens Chloride has a -1 charge Pent means it has 5 oxygens SnCl4 N2O5