Naming Chemical Compounds PPT
advertisement

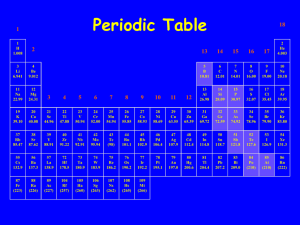
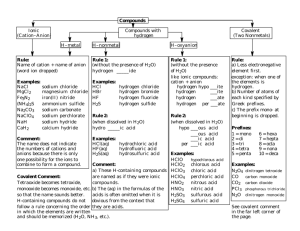
Naming Chemical Compounds Chemical Nomenclature From the Latin: Nomen – name calare – to call 10 million known chemicals Naming Ionic Compounds Naming Rules Cations Rule 1: Cations formed from metal ions have the same name as the metal.( positive ion) Write the name of the first element Example K+ potassium Zn2+ zinc Rule 2: Cations that form can form different charges, use a Roman numeral Example Fe2+ iron(II) Fe3+ iron(III) Use the table on p364 in your text book! Anions Rule 3: Anions( negative ion) drop the ending of the elemental name and add ------- ide. Example Hhydride O2- oxide Rule 4: Polyatomic anions containing oxygen have names ending in -----ate or -------ite Use the table on p366 in text book (you do not have to memorize these) Example NO3- nitrate Remove 1 oxygen atom NO2- nitrite a.k.a. oxyanions Naming the Ionic Compound Cation - Anion BaBr2 Al(NO3)3 Al(NO2)3 CuSO4 Mg(ClO4)2 Fe(HCO3)3 barium bromide aluminum nitrate aluminum nitrite copper(II) sulfate magnesium perchlorate iron(III) hydrogen carbonate Let’s Go The Other way potassium sulfide calcium hydrogen phosphate nickel(II) hypochlorite sodium peroxide strontium nitride sodium oxide ammonium sulfide K2S CaHPO4 Ni(ClO)2 Na2O2 Sr3N2 Na2O (NH4)2S Special Cases for oxygenated halogens Add 1 extra oxygen Base Less 1 oxygen Less 2 oxygen ClO4ClO3ClO2ClO- perchlorate chlorate chlorite hypochlorite ****Use for FO3-, ClO3-, BrO3-, and IO3- Rule 6: Adding H+ to an oxyanions. CO32- carbonate HCO3- hydrogen carbonate PO43HPO42H2PO4- phosphate hydrogen phosphate dihydrogen phosphate H+ reduces the charge Naming Inorganic Acids Acid – A substance which yields hydrogen ions(H+) when dissolve in water. All acids will begin with an “H” as the first element. Example: HNO3 HCl Rule 1: Acids based on anions whose name ends in -------ide uses hydro as a prefix and -----ic as a suffix Example HCl hydrochloric acid H2S hydrosulfuric acid ***These types of acids are called binary acids. Rule 2: Acids based on anions whose names in -----ate uses -----ic as a suffix Example HClO3 chloric acid H3PO4 phosphoric acid Rule 3: Acids based on anions whose name ends in -----ite uses -----ous as a suffix Example HClO2 chlorous acid H2SO3 sulfurous acid Naming Binary Molecular Compounds ( covalently bonded atoms-nonmetals) Rules 1. The first shown element is named as the element( example-CO---carbon is the first element) 2. The name of the second element is given an -----ide ending. 3. The second element always carries a prefix indicating the # of times it is present-example CO2 dioxide. 4. The amount of the first element is only shown if it is present more than once. 4. Greek prefixes are used to indicate the number of atoms of each element mono 1 di tri tetra penta hexa hepta octa nona deca 2 3 4 5 6 7 8 9 10 5. The prefix “mono” is never used with the first element. 6. When a prefix ends in “a” or “o” and the name of the second element begins with a vowel, the “a” or “o” is often dropped. Examples OCl2 Oxygen dichloride NF3 P4S10 SO2 PCl5 N2O3 Nitrogen trifluoride Tetraphosphorus decasulfide Sulfur dioxide Phosphorus pentachloride Dinitrogen trioxide The other way Silicon tetrabromide Disulfur dichloride Triphosphorus monoxide SiBr4 S2Cl2 P3O