Writing and Naming Molecular Compounds
advertisement

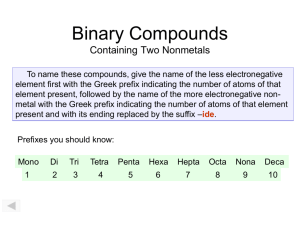
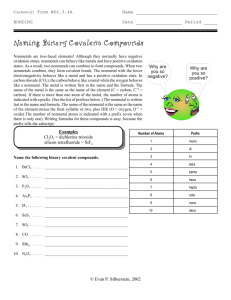
Writing and Naming Binary Molecular Compounds • Composed of two anions (binary) • Check on periodic table to make sure! • Name describes the type and number of the atoms in a molecule of the compound. • The most metallic (farthest left element or lowest) is written 1st. • Write the second element changing the ending to -ide. • Be sure to place Greek prefixes before each element to indicate the actual number of atoms in the molecule. Never simplify! • Note: Never place “mono” on the first element Greek prefixes • • • • • • • • • • 1- mono 2- di 3- tri 4- tetra 5- penta 6- hexa 7- hepta 8- octa 9- nona 10- deca • • • • • • • • CO carbon monoxide CO2 carbon dioxide N 2O 5 dinitrogen pentoxide SF3 sulfur trifluoride Molecular Names to Formulas • Disulfur heptachloride • Xenon trioxide • Dinitrogen tetroxide • Sulfur hexachloride • Carbon disulfide • Oxygen difluoride