Nomenclature Flowchart
advertisement

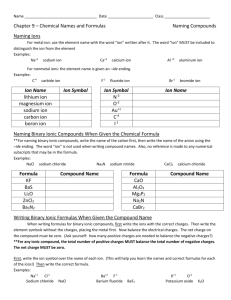
Nomenclature Flowchart Answer the questions, find the rules, name the compound or determine the formulas. Is the compound 2 elements only – one is a metal? • Is the metal group 1, 2, or Al, Zn, Ni, Ag? • Rule- metal gets metal name, nonmetal gets nonmetal name with …ide ending. Balance charges. • • • • • Examples KCl Potassium chloride Li2S Lithium sulfide MgF2 Magnesium fluoride ZnO Zinc oxide Or is it a transition or other metal? Rule- metal gets metal name, (Roman numeral for the charge of the metal atom), non metal gets nonmetal name with ide ending. Balance charges. Examples: CuBr Copper(I) bromide FeO Iron(II) oxide Au3N Gold(I) nitride SnI2 Tin(II) iodide Is the compound more than 2 elements, one is a metal or ion? • The compound contains a polyatomic ion. • Polyatomic ions must be memorized by name, chemical formula and charge. • Rule- metal gets the metal name,(charge if transition metal) followed by polyatomic ion name. Balance charges. • If polyatomic is the cation, than polyatomic ion name first, followed by nonmetal with ide ending, or name of the second polyatomic ion. Balance charges. Poly atomic ion examples: • • • • Na2SO4 Ca(ClO2)2 Cr2CO3 Pb(OH)4 Sodium sulfate Calcium chlorite Chromium(I) carbonate Lead(IV) hydroxide Is the compound 2 elements onlyboth are non metals? • Rule- first element gets nonmetal name, second element gets the ide ending. • Use prefixes to indicate number of each non metal atom in the compound. • Exception – if compound contains only one atom of the first element the mono is omitted. • Prefixes-Mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca. 2 non metal examples • • • • P2S5 CCl4 IF3 N2O Triphosphorous pentasulfide Carbon tetrachloride Iodine trifluoride Dinitrogen monoxide Are you given formula and you must provide the name? • Look at the name closely- it will follow the same format of either cation followed by anion or will have prefixes (slide 4). • If cation /anion- metal name for the cation, anion will be metal name if -ide ending, or polyatomic ion if it has -ate or -ite ending. • Remember to balance the charges. Naming Acids - recognized by H first element or in water (aq) • Rule- acid with no O atoms • Hydro_____ic acid • Rule –acid with O atom(s)- named for the polyatomic ion. • If –ate polyatomic than ______ic acid • If –ite polyatomic than ______ous acid. Acid examples • • • • HCl HCN HNO2 H3PO4 Hydrochloric acid Hydrocyanic acid Nitrous acid Phosphoric acid