Chapter 5 - Green Local Schools
advertisement

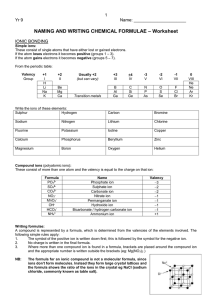
Ionic Compounds Ch.5 (5-1) Ions • Atom or group of atoms that has a charge b/c it has lost or gained e• Ex: [Na] = 1s22s22p63s1 [Na+] = 1s22s22p6 Types of Ions • Cation: + ion – Lose e– Ex: K+ • Anion: - ion – Gain e– Ex: Br- Terms • Electroneutrality: having = #’s of + & charges • Isoelectronic: having the same econfig. as another atom – [Na+] = 1s22s22p6 = [Ne] Octet Rule • Tendency of atoms to gain or lose e- so that their outer s & p orbitals are full w/ 8 e- Transition Metal Ions • Form cations • Some have multiple ions due to d orbitals – Ex: Fe2+ = Iron (II) Fe3+ = Iron (III) Ionic Compounds • Chemical cmpd composed of oppositely charged ions • Binary ionic cmpd: cation of 1 element & anion of another – Ex: ZnS, KBr Naming Binary Ionic Cmpds • Name of cation – Ca = calcium • Name of anion – S = sulfur • Drop anion suffix & add –ide – CaS = calcium sulfide Naming Practice • Al2S3 – Aluminum sulfide • Rb2O – Rubidium oxide • FeF2 – Iron (II) fluoride • CrI3 – Chromium (III) iodide Specify charge of transition metals Writing Formulas • Write the symbol & charges for the cation & anion – Aluminum oxide: Al3+O2- • Balance the charges by adding subscripts – Al2O3 Formula Practice • Calcium oxide – CaO • Potassium nitride – K3N • Tin (II) oxide – SnO • Copper (I) bromide – CuBr (5-2) Ionic Bond • Force of attraction b/w ions of opposite charge Terms • Coulombic force: attraction or repulsion b/w 2 charged objects • Halide: salt w/ halogen anion – Ex: NaCl & KBr Crystal Lattice • Repetitive geometric arrangement of atoms • Unit cell: smallest repeating unit in a crystal NaCl Lattice Energy • E released when atoms, ions, or molecules come together to form a crystal Properties of Binary Ionic Cmpds • Hard & brittle – From crystal lattice • Melt & boil at high temps. – Need lots of E to break bonds • Solids generally don’t conduct electricity (5-3) Polyatomic Ion • Group of bonded atoms that functions as a single ion • Ex: NO2- = nitrite SO42- = sulfate Oxyanions • Neg. polyatomic ion containing oxygen • Suffix: – -ate, most common ion • sulfate: SO42- – -ite, anion w/ 1 less O • sulfite: SO32- Oxyanions (cont.) • Prefix: – Hypo-, anion w/ 1 less O than –ite – Per-, anion w/ 1 more O than -ate • Ex: hypochlorite = ClO-, chlorite = ClO2chlorate = ClO3perchlorate = ClO4- Naming • Name the cation – K+ • Name the anion – CO32- • Name the salt – K2CO3 = potassium carbonate Polyat. Ion Naming Practice • NaOH – Sodium hydroxide • LiClO2 – Lithium chlorite • H 2O 2 – Hydrogen peroxide • CaCO3 – Calcium carbonate Writing Formulas • Determine formula & charge of cation – Calcium = Ca2+ • Determine formula & charge of anion – Chlorite = ClO2- • Balance charges (keep polyat. ion in parentheses if necessary) – Calcium chlorite = Ca(ClO2)2 Polyat. Ion Formula Practice • Iron (II) hydroxide – Fe(OH)2 • Potassium dichromate – K2Cr2O7 • Aluminum phosphate – AlPO4 • Ammonium nitrate – NH4NO3 Oxidation #’s • # assigned to an atom in a polyatomic ion or molecular cmpd based on an assumption of complete transfer of e- Assigning Oxid. #’s • The sum of the oxid.#’s for all the atoms in a cmpd = 0 • The sum of oxid.#’s for all atoms in a polyatomic ion = charge on that ion Assigning Oxid. #’s (cont.) • Free (uncombined) elements = 0 – Na, O2 • Monatomic ion = charge of its ion – K+ = +1 • More EN element in binary cmpd = its charge if it were an ion – NaCl: Cl = -1 Assigning Oxid. #’s (cont.) • H = +1 – w/ a metal it’s –1 • F = -1 • O = -2 – w/ F it’s +2 – in peroxides (H2O2) it’s –1 • In cmpds, Gr. 1 & 2 & Al are +1, +2, & +3, respectively Oxidation # Practice • Determine K in KOH • -2 +1 – KOH =0 K = +1 • -2 +1 • Determine Cl in Ca(ClO3)2 • +2 – -2 Ca Cl2 O6 = 0 • +2 -12 Cl = +10 = +5 2 Oxidation # Practice • Determine N in NO3• -2 – N O3 - = -1 N = +5 • -6 • Determine N in NH4+ • +1 – N H4 + = +1 • +4 N = -3 Hydrate • Ionic cmpd that contains water molecules in its crystal lattice – Naming: use prefixes (See Table 5-8) • Na2CO3•10H2O = sodium carbonate decahydrate • Anhydrous: w/out water Hydrate Prefixes • • • • • Mono - 1 Di - 2 Tri - 3 Tetra - 4 Penta - 5 • • • • • Hexa - 6 Hepta - 7 Octa - 8 Nona - 9 Deca - 10 Naming Hydrates Practice • BaSO4•5H2O – Barium sulfate pentahydrate • MgSO4•7H2O – Magnesium sulfate heptahydrate • SrCl2•2H2O – Strontium chloride dihydrate