Chemical Reactions: Collision Model & Activation Energy
advertisement
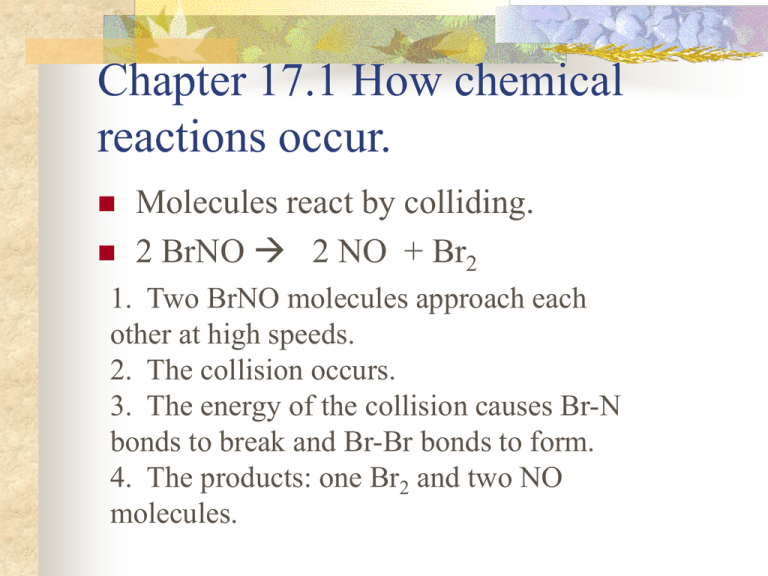
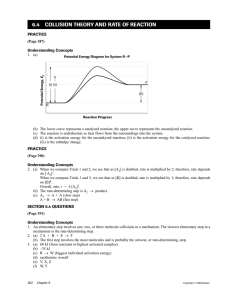
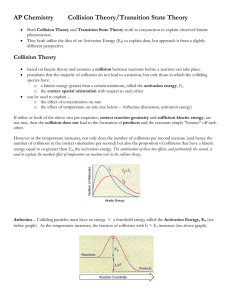
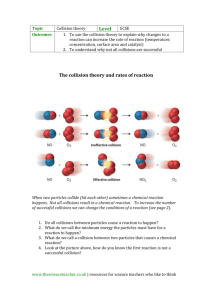
Chapter 17.1 How chemical reactions occur. Molecules react by colliding. 2 BrNO 2 NO + Br2 1. Two BrNO molecules approach each other at high speeds. 2. The collision occurs. 3. The energy of the collision causes Br-N bonds to break and Br-Br bonds to form. 4. The products: one Br2 and two NO molecules. Collision Model Collision Model = Reactions occur during molecular collisions. The reaction rate is directly related to the number of collisions. This model explains why a reaction proceeds faster if the concentrations of the reacting molecules are increased or the temperature is increased. 17. 2 Activation energy Activation energy (Ea) = minimum energy needed for a reaction to occur (at higher temperatures, the average collions have more energy) 17.2 Catalyst Catalyst = a substance that speeds up a reaction without being consumed. Enzymes are catalysts in your body. They allow bodies to speed up complicated reactions that would be too slow to sustain life at normal body temperatures. Because of the lower activation energy, more collisions will have enough energy to allow a reaction. Ozone The breakdown of ozone (O3) is catalyzed by chlorine atoms. One chlorine atom can catalyze the destruction of about 1 million ozone molecules per second. Cl + O3 ClO + O2 O + ClO Cl + O2