Chemical Reaction Rates: Factors & Experiment
advertisement

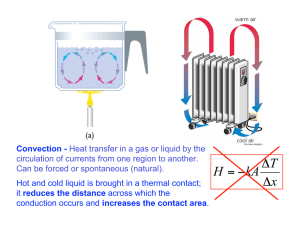
SNC 2D1 Factors Affecting the Rate of a Chemical Reaction The speed at which a chemical reaction occurs is called the rate of reaction. For example, a log in a campfire undergoing combustion (burning) can either smolder slowly for several hours or rapidly burn down to ash. What controls the rate of this chemical reaction? The Kinetic Molecular Theory The particle theory of matter tells us that as molecules are heated, they move more rapidly. In solids, particles do not move freely since they are held together by attractive charges. In liquids, molecules move freely and slide over each other but remain at the bottom of a container. In a gas, the particles are far apart and can move freely in a straight line. Solid Liquid Gas The collision model of chemical reactions states that the rate of a chemical reaction is affected by the number of collisions between reactant molecules. If a molecule is moving with enough speed (kinetic energy) and collides with another molecule, the reaction can take place. Therefore, the rate of reaction is controlled by: 1) the number of collisions. 2) the fraction of effective collisions. 1. Temperature As the temperature of a substance is raised, the speed or kinetic energy of the molecules within that substance increases. This increases results in1) more collisions and 2) a larger fraction of successful collisions. Therefore as we raise the temperature of a substance undergoing a chemical reaction, we increase the reaction rate. E.g. For example, compare the reaction of iodide with starch. 1 2 Temperature = __________ oC Temperature = ___________ oC Reaction time = _________ sec. Reaction time = __________ sec. 2. Concentration As we increase the concentration of reactants in a chemical reaction, we pack more molecules within the same volume (e.g. grams/L). Putting more molecules in the same volume increases the chance of collisions and therefore increases the rate of reaction. Reactant concentration = 1% Reactant concentration = 2% Reaction time = _________ sec. Reaction time = __________ sec. 3. Surface Area Surface area is the area of sample of matter. When reactants of different phases (e.g. solid & gas, liquid & gas, solid & liquid) are in contact, the surface area affects the number of possible collision sites between reactants. For example, grinding a solid into a fine powder can greatly increase the surface area and speed up a chemical reaction. Large pieces of starch SA = 1 cm2 x 6 = 6 cm2 Smaller grains of starch SA = 0.25 cm2 x 6 x 8 =12 cm2 Longer reaction time (i.e. slower) Shorter reaction time (i.e. faster) 4. Catalysts A catalyst is a substance that increases the rate of reaction without being consumed in the reaction. Catalysts can lower the collision energy required for a chemical reaction to occur. This increases the fraction of reactant collisions that lead to a reaction. Catalysts are very important in industry and pollution control. A “catalytic converter” is required on all automobiles to complete the combustion reaction and prevent the release of many smog-forming molecules. Blast Off! In your final chemistry experiment, you will be manipulating the first three variables (Temperature, Concentration and Surface Area) to optimize the reaction of Alka-Seltzer tablets. The goal is to blast the lid off a film canister in precisely 15 seconds. In groups of 3, complete Design steps a) to d) on p.276. Identify your independent and dependent variables. Have your design approved before you leave class. Homework: Questions p264 #1-5, 7-10