Activation energy:
advertisement

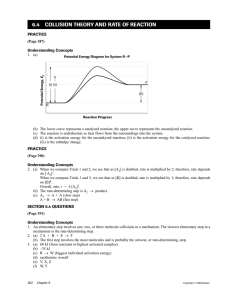
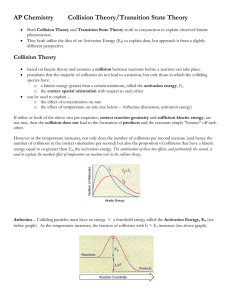
Activation energy: there are two theories about the activation energy:1.collision theory: according to the collision theory ,the reaction occurs when the reactants molecules A and B come very close to each other or collide. Only those collisions result in product formation in which the colliding molecules are associated with a certain minimum amount of energy . this minimum energy which the molecule should possess so that their mutual collision result in a chemical reaction is called "threshold energy". Collisions among molecules possessing energy less than threshold energy are not effective collisions and do not result in the formation of products. The additional energy required by the molecule to attain the threshold energy is called activation energy. 2.transition state theory: the molecules must be in an activated state before they can react. There is a minimum energy level denoted by Ex to whuch the reactant molecule must be raised to enable it to undergo a chemical change. The activation energy is the energy required to form the activated complex or intermidate. Effect of temperature on rate of reaction:there is a significient relationship between the rate of chemical reaction and the temperature at which the reaction takes place. For homogeneous reactions ,it is usually found that the velocity constant of the reaction is approximately doubled for every ten degree rise in temperature and the realationship can be represented by Which is Arrhenius equation.