AP Chemistry
advertisement

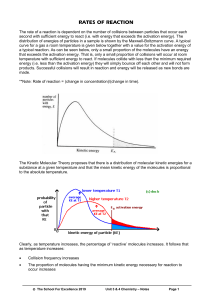
Honors Chemistry Name: ______________________________________ Date: _______________ Mods: ___________ Ch. 14 – Chemical Kinetics: The Basics 1. Define chemical kinetics – Examples: o How quickly will medicine begin working? o How quickly is the ozone being depleted? o How long has this fossil been preserved? 2. What will impact the rate of a reaction? All chemical reactions involve… o Breaking up reactant bonds o Forming new product bonds As a result, the __________________ (identity & state) of the reactants plays a huge role in reaction rates 14.1 - Factors that Affect Reaction Rates 3. Four Factors that affect reaction rates: 1) Physical State of the Reactants: o molecules must ________________ in order to react… o more collisions = ________________ rate or reaction o Solids: Ground up solids = faster rate of reaction More _________________ _________ of reactants exposed = greater likelihood of collisions o Homogeneous Mixtures: gas or liquid solutions react faster because particles are constantly colliding with greater kinetic energy than solids for gases, increasing _______________________ = more collisions = faster reaction rate 2) Concentration of Reactants: o ____________________ concentrations have more particles present in solution More particles= more collisions = faster reaction rate 3) Temperature: o ____________________ temperatures = greater kinetic energy Faster moving particles = increased # of collisions & with higher energy = faster reaction o Example: glowsticks & chemiluminescence (light generated by a chemical change) 4) Presence of a Catalyst: o Define catalyst – . Catalyst itself does NOT ________________ (it’s not used up in the actual chemical reaction) Think of catalysts like “matchmakers” bringing reactants together faster MnO2 is the catalyst in this reaction (given over the yield arrow) Cute Video: http://ed.ted.com/lessons/how-to-speed-up-chemical-reactions-and-get-a-date 14.2 - 14.3 – Reaction Rates & Concentrations 4. Reaction rates can be determined by monitoring the change in _________________________ of either reactants or products as a function of time. For the reaction, A B… o At the beginning of the reaction (t = 0 sec.), 100% of the particles are reactants (higher concentration of molecule A) o Near the end of the reaction, the majority of the particles are products (higher concentration of molecule B) For the rxn, C4H9Cl (aq) + H2O (l) C4H9OH (aq) + HCl (aq) o On this reaction, the concentration of the reactant, C4H9Cl was measured as time pases o The rate at which the reaction progresses is a factor of the concentration or reactant over time 14.4 – Temperature & Rate 5. The rate of most chemical reactions ___________________ as temperature rises. Why does temperature have such a significant role in reaction kinetics??? ... because of the collision model 6. The Collision Model: Rxns only occur when reactants collide (reactant bonds are broken & product bonds can form) Trouble is… any old collision won’t due … For molecules to react, they must have an “________________________” collision o For “effective” collisions to occur between molecules, there are two requirements: (1) Molecules collide with each other in the correct ____________________________ (2) Molecules collide with _____________________ _________________ to cause bond breakage and product formation The Collision Model: Molecular Orientation o Molecules interacting are like ___________________ pieces: o Given, A + B AB o Only 1 part of molecule A will react with molecule B to form an “effective” collision o If you want to form product AB, A & B must align (or be oriented) correctly o Increasing the _____ of collisions = greater chance of having an effective collision The Collision Model: Energy o Okay… so you have a collision where the molecules are aligned correctly (puzzle pieces fit)…. But they still might __________ react if they don’t have a high enough ____________________ energy to break pre-existing reactant bonds… o So how much energy does it take??? The amount is different for every reaction, but it is known as the _______________________ energy (Ea) of the reaction Define activation energy (Ea) – Analogy: × In golfing, if the ball does not have enough energy, it can’t make it over a hill to even get into the hole × o If molecules in a rxn don’t have enough energy to get over the activation energy barrier, the reaction can’t occur to even form products Energy Diagram & Activation Energy 14.7 – Catalysts 7. Catalysts So how does a catalyst speed up a reaction WITHOUT being used up??? Catalysts _____________________ the activation energy required for “effective” collisions o 8. This means a ______________________# of collisions will be considered “effective” = more molecules reacting = faster reaction rate Catalysts in your body!!! In Biology, catalysts involved with reactions in living things are known as __________________________ (very selective molecules that specifically target other molecules – known as “__________________________” – in the human body) Enzyme has an activation site which only fits particular substrates - fit together like a “lock & key”